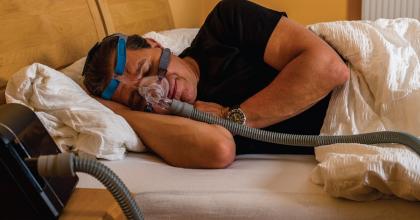
Consumers (Medical Devices)
Sub-Topic Paragraphs
Popular Topics
Paragraph Header
Contact Us
Paragraph Header
Contact Us
Contact Point
Division of Industry and Consumer Education
Center for Devices and Radiological Health
Food and Drug Administration
10903 New Hampshire Ave
Silver Spring, MD 20993
Center for Devices and Radiological Health
Food and Drug Administration
10903 New Hampshire Ave
Silver Spring, MD 20993
(800) 638-2041
(301) 796-7100
Hours Available
Office of Communication, Information Disclosure, Training and Education
For Latest Updates Follow
Contact Point Twitter
@FDADeviceInfo
Information and news on device recalls, other safety issues, approvals, and other device and radiation-emitting product topics.