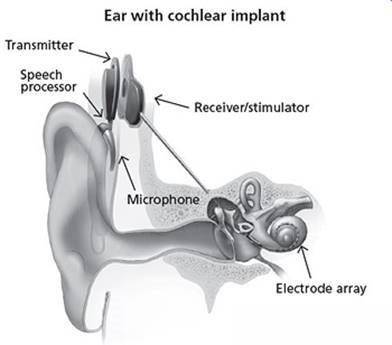
Cochlear Implants
Cochlear implants are electronic hearing devices. Doctors implant cochlear implants into people with severe to profound hearing loss to produce useful hearing sensations.
The FDA regulates manufacturers of cochlear implants. For manufacturers to sell cochlear implants in the United States, they must first show the FDA that their implants are safe and effective. As a matter of policy, the FDA does not rate or recommend brands of cochlear implants or medical facilities that implant them.
In this section:
- What Is a Cochlear Implant?
- Benefits and Risks of Cochlear Implants
- Frequently Asked Questions
- FDA-Approved Cochlear Implants
- What Educators Need to Know
- Before, During, and After Implant Surgery
- MRI for Patients with Cochlear Implants