COVID-19 Test Basics
Have a COVID-19 test you think may be expired? Read more about expiration date information for FDA-authorized at-home OTC COVID-19 diagnostic tests.
COVID-19 testing plays a critical role in the fight against the virus. Understanding COVID-19 tests, including the different types of tests and their uses, and the types of samples the tests use, is key to making an informed decision that meets your needs.
Types of Tests
There are different types of COVID-19 tests – diagnostic tests and antibody tests.
Diagnostic tests can show if you currently are infected with SARS-CoV-2, the virus that causes COVID-19. There are two common types of COVID-19 diagnostic tests:
- Molecular tests, such as polymerase chain reaction (PCR) and other nucleic acid amplification tests (NAATs) tests, which detect genetic material called RNA from the virus
- Antigen tests, often referred to as rapid tests or, for some, at-home or self tests, which detect proteins called antigens from the virus
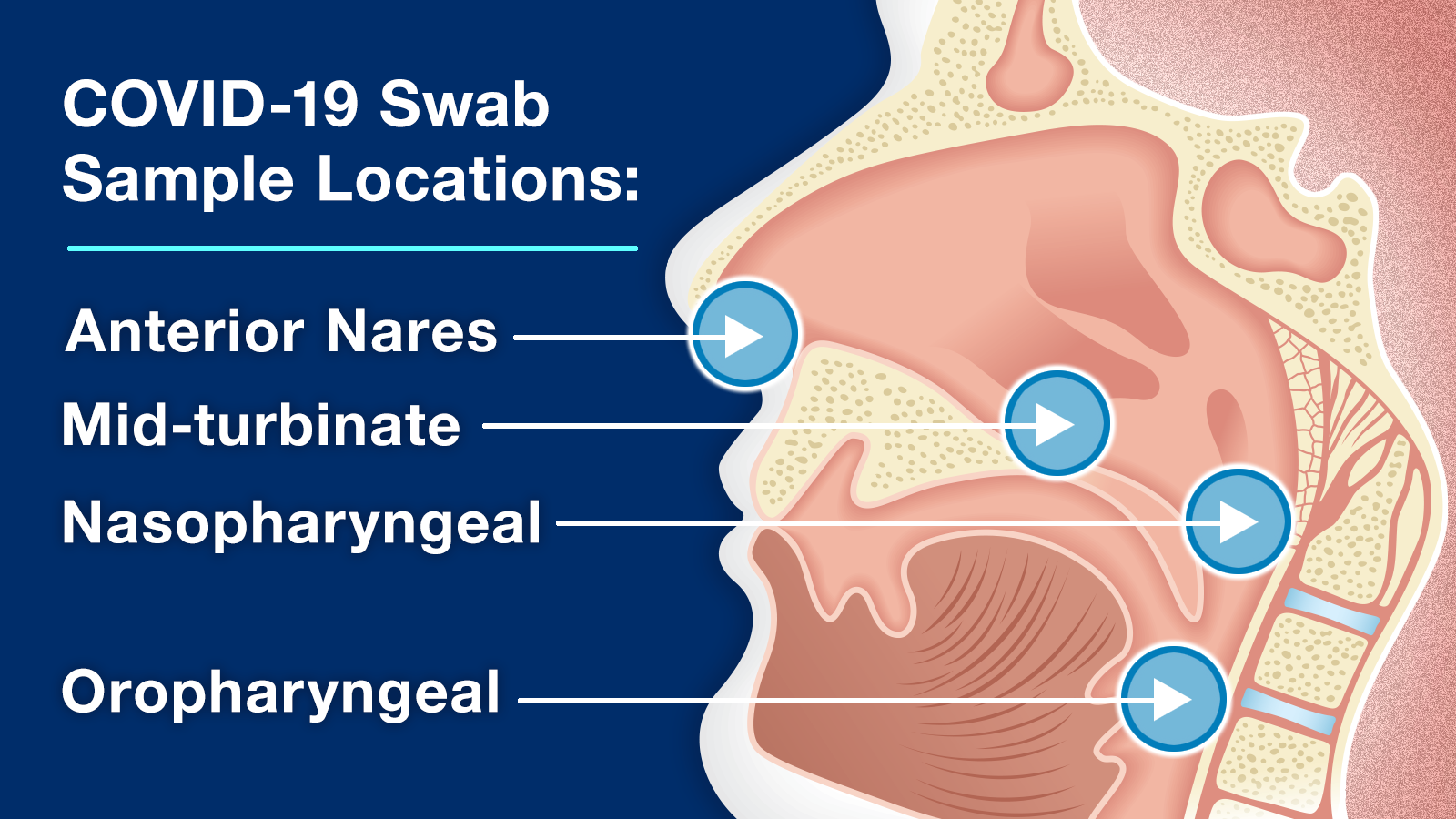
Samples for COVID-19 diagnostic tests are typically collected using an anterior nares (nasal) swab sample. Some diagnostic tests use other samples such as mid-turbinate, nasopharyngeal, oropharyngeal, or saliva samples. Depending on the intended use, COVID-19 diagnostic tests can be performed at a laboratory, a standalone testing site, a doctor’s office or health clinic, or at home. For most molecular COVID-19 diagnostic tests, you go to a testing site to have your sample collected and for others you can collect your own sample at home using a home collection kit and mail it to a laboratory for testing. Some tests, including most antigen tests, can be performed completely at home, giving you results within minutes, without needing to send your sample to a laboratory.
If you think you need a COVID-19 diagnostic test, you can find a community testing site in your state. You can also use an FDA-authorized at-home COVID-19 diagnostic test which gives you the option of self-testing where it is convenient for you. Be sure to check the At-Home OTC COVID-19 Diagnostic Tests website for information on expiration dates, who can use the test, and other details that may help you decide what test is right for you. Be aware that COVID-19 diagnostic tests are authorized for specific uses and that laboratory-based molecular COVID-19 tests, are generally more accurate than at-home tests.
To increase the accuracy of an at-home COVID-19 antigen diagnostic test, it is important to perform repeat testing, after 48 hours, following a negative test result, whether you have symptoms or not, to reduce your risk of a false negative test result. For more information about how to reduce your risk of getting a false negative result on an at-home COVID-19 antigen test, read our FDA Safety Communication. For additional information on reading and understanding your test results, see Understanding At-Home OTC COVID-19 Antigen Diagnostic Test Results.
For details about each authorized COVID-19 diagnostic test, see the lists of authorized Molecular Diagnostic Tests and Antigen Diagnostic Tests, as well as the At-Home COVID-19 Diagnostic Tests webpage. Using the search box in the EUA tables, you can use keywords to search and filter the type of test or collection kit you are looking for. As new tests are authorized for use, they are added to these tables so that anyone can access up-to-date information on all authorized tests and collection kits.
Antibody (or serology) tests look for antibodies in your blood that your immune system produced in response to SARS-CoV-2, the virus that causes COVID-19. Antibody tests should not be used to diagnose a current SARS-CoV-2 infection or COVID-19 and, at this time, should also not be used to check for immunity. More research is needed to determine what, if anything, antibody tests can tell us about a person’s immunity.
Samples for antibody tests are typically collected by a doctor or other medical professional by taking blood from a finger stick or your vein. For more information about antibody testing, visit Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers.
Types of Samples
Different tests are authorized to be used with different types of samples. The most common sample types are:
Swab samples use a swab (similar to a long Q-Tip) to collect a sample from the nose or throat. The types of samples include:
- Anterior Nares (Nasal) – takes a sample from just inside the nostrils
- Mid-turbinate – takes a sample from further up inside the nose
- Nasopharyngeal – takes a sample from deep inside the nose, reaching the back of the throat, and should only be collected by a trained health care provider
- Oropharyngeal – takes a sample from the middle part of the throat (pharynx) just beyond the mouth, and should only be collected by a trained health care provider
Saliva samples are collected by spitting into a tube rather than using a nose or throat swab.
Blood samples are only used to test for antibodies and not to diagnose COVID-19. Venous blood samples are typically collected at a doctor’s office or clinic. Some antibody tests use blood samples from a finger stick.
Read More
You can read more about the individual types of tests, safety communications and how to interpret your test results at the links below:
- FDA authorized molecular tests
- FDA authorized antigen tests
- At-home antigen test page
- Safety communication
- Understanding at-home OTC antigen test results
Report Adverse Events
The FDA encourages health care professionals and patients to report adverse events or side effects as well as performance issues related to the use of COVID-19 tests or other medical products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report online through the FDA’s MedWatch website.
- Download the form or call 1-800-332-1088 to request a form, then complete and return to the address on the form or submit by fax to 1-800-FDA-0178.