Focus Area: Novel Technologies to Improve Predictivity of Non-clinical Studies and Replace, Reduce, and Refine Reliance on Animal Testing
Importance to FDA
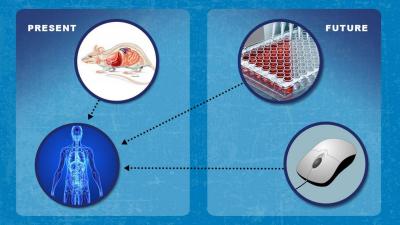
Animal studies are frequently used to assess certain aspects of risk, toxicity, activity, or public health impact of FDA-regulated products that focus on a product’s nature, chemistry, effects (pharmacology), and its potential damage to the body (toxicology). FDA is working to replace, reduce, and refine (the 3 Rs) dependence on animal studies by advancing development of, and evaluating new, fit-for-purpose non-clinical tools, standards, and approaches that may someday improve predictability. In some cases, in silico modeling, such as using available information in computational science approaches to predict safety issues, can be used to supplement and may potentially replace some risk analyses that are currently based on animal data. FDA uses non-clinical and clinical data to assess the products it regulates. The predictive value of current non-clinical testing approaches varies. Sometimes clinical evaluations identify risk and safety concerns that were not predicted by current non-clinical methodologies. Therefore, the development of tools with improved predictive value may enable FDA to make informed decisions about clinical investigations, licensures, and other all FDA regulated products such as food, cosmetics, and tobacco.
The development and eventual availability of new alternative methods may more accurately identify, predict, evaluate, and reduce the degree and likelihood of risks of FDA-regulated products. In addition to enhancing safety, this could also help speed development and reduce costs in assessing new FDA-regulated products, leading to improved health outcomes. Systematically assessing and comparing the information from alternative methods with traditional methods offers an opportunity to evaluate the applicability and predictability of the new approaches and their ability to support FDA's regulatory mission of safeguarding public health.
Examples
- Developmental neurotoxicity tests are needed especially for sensitive populations such as infants and children. Neurotoxicity occurs when the nervous system (e.g., brain) is purposely or accidentally exposed to toxic substances such as chemotherapy, lead, and mercury. FDA is actively exploring alternative methods such as using in vitro cultures of developing brain stem cells since there is no currently established predictive in vitro neurotoxicity assay that allows correlation to neurodevelopment in pediatrics.
- FDA participates in large multi-laboratory studies to assess the reliability, sensitivity, specificity, and reproducibility of in vitro alternatives to in vivo assays (e.g., to assess potency of certain vaccines).
- The Open Online Simulations for Stimulating Peripheral Activity to Relieve Conditions (o2S2PARC) accelerates the availability of safe and effective neuro-prosthetics. FDA developed a freely accessible online platform that enables developers to simulate modulation of the peripheral nervous system and its impact on organ physiology using validated models and state-of-the art in silico tools.
- Investigators in multiple centers are working with microphysiological systems either produced commercially, in house, or by academics to assess their performance and applicability to toxicity and activity assessment.
- FDA, through the International Cooperation on Cosmetics Regulation (ICCR), has been involved in numerous activities to address new alternative methods (MAMS) to assess cosmetic safety.
| Minority Health and Health Equity | Women’s Health | Maternal Health | Pediatric Health |
| Oncology | Rare Diseases | One Health Initiative |
Research Capabilities, Tools, and Resources
| Research Management and Collaborations | Technology Transfer and Public-Private Partnerships | Physical Standards and Reference Materials | Intramural Grant Programs | Extramural Funding Mechanisms |
Scientific Education, Training, and Communication
| Fellowship and Training Opportunities | Professional Development and Continuing Education | Communication and External Meetings |
Infrastructure
| Facilities and Shared Resources | Safety and Compliance |
| Office of the Chief Scientist | Contact Us: FARS@fda.hhs.gov |