Importing Medical Devices
Overview
This page provides an overview of medical devices and the requirements that the FDA verifies/enforces at the time they are imported or offered for import into the United States.
The Center for Devices and Radiological Health (CDRH) is the FDA center responsible for overseeing the medical device program. Visit the Medical Devices webpage for more information.
- Overview
- Registration and listing
- Premarket submissions
- Medical device classification
- Affirmation of Compliance codes for medical devices
- Combination products
- Personal importation of medical devices
- Decorative contact lenses
- Device labeling
What is a medical device?
The FDA defines a medical device as:
- "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part or accessory which is: recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
- intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
- intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.
- The term ‘‘counterfeit device’’ means a device which, or the container, packaging, or labeling of which, without authorization, bears a trademark, trade name, or other identifying mark or imprint, or any likeness thereof, or is manufactured using a design, of a device manufacturer, processor, packer, or distributor other than the person or persons who in fact manufactured, processed, packed, or distributed such device and which thereby falsely purports or is represented to be the product of, or to have been packed or distributed by, such other device manufacturer, processor, packer, or distributor."
Is my product a medical device?
If your product is labeled or used in a manner that meets this definition it will be regulated as a medical device and is subject to the FDA’s laws and regulations before, during, and after it is offered for sale or use in the United States. View examples of medical devices.
To assist you in determining if your product is a medical device, you may visit the “Is the Product a Medical Device?” page.
Additionally, the Federal Food, Drug, and Cosmetic Act (the Act) provides a means for obtaining the FDA’s views about the classification and the regulatory requirements that may be applicable to your particular device. For more information visit the Guidance for Industry and FDA Staff on Procedures for Section 513(g) Request for Information.
If your device is also a radiation-emitting electronic product there may be additional requirements. Please visit our radiation-emitting electronic products page for more information.
What medical device requirements are verified at the time of importation?
At the time of importation, the FDA will verify compliance with the following requirements as applicable:
- Registration
- Listing
Additionally:
- Certain medical devices may need to comply with Premarket Submission requirements (Premarket Notification or Premarket Approval).
- The FDA conducts field examinations and analyzes samples of medical devices to ensure they comply with applicable standards and/or label requirements.
- The FDA checks the import alert database to ensure the manufacturer or product is not subject to detention without physical exam (DWPE) and listed on an import alert. For example, import alert 89-04 lists foreign manufacturers not in compliance with Medical Device Good Manufacturing Processes .
How does the FDA verify compliance with the medical device requirements?
The FDA entry reviewers are trained to verify compliance with applicable product requirements using the information provided to the FDA in the importer’s entry transmission such as:
- Declared manufacturer
- Declared importer/consignee
- Product description
- Affirmations of Compliance (A of C)
These entry declarations are compared to information in the FDA’s internal data systems. The FDA uses the internal data systems to verify registration, listing, device approval (when required) or other product requirements and to determine if the firm is subject to DWPE. If the information submitted matches, then compliance is verified; if the information submitted does not match, the FDA may gather additional information or may detain the product.
The submission of correct and accurate entry data along with the relevant A of C codes will help expedite the entry review process. Supplying this information accurately increases the likelihood that your shipment will be processed electronically and not held for manual review because the FDA’s screening tool, PREDICT, can verify the declared information against the FDA's internal data systems.
Note: Submitting inaccurate or incomplete information may delay the review of your entry.
Registration and listing
Establishments that are involved in the production and distribution of medical devices intended for commercial distribution in the United States are required to register annually with the FDA. Most establishments that are required to register are also required to list the devices and the activities performed on those devices at that establishment.
For more information on registration and listing, visit the following page: Who Must Register, List and Pay the Fee.
How does the FDA verify registration and listing?
When an entry is transmitted to the FDA, we verify that the declared manufacturer and shipper are registered with the FDA by comparing the submitted information with CDRH’s establishment registration and listing database. We also use CDRH’s data system to verify that the declared importer is registered.
Listing for the declared product is also verified by comparing the declared product description to CDRH’s establishment registration and listing database.
If the information submitted matches the CDRH establishment registration and listing database, then compliance is verified; if the information does not match, the FDA may gather additional information or may detain the product. If a firm lacks the required registration and listing, the product will be subject to refusal.
How do I obtain the manufacturer's registration and listing information?
You may search the medical device registration and listing database for registration information for any medical device firm that is registered with the FDA. When applicable, the database also includes devices listed with the FDA. In order to obtain the listing number, you will have to contact the firm that listed the device.
Premarket submissions
Some medical devices require premarket submissions depending on use and classification:
- Premarket Notification (510(k))
A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is safe and effective, and substantially equivalent to a legally-marketed device that is not subject to Premarket Approval. Visit our Premarket Notification 510(k) page for more information.
- Premarket Approval (PMA)
This is the FDA’s process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. Visit our Premarket Approval page for more information.
To determine if your device requires a 510(k) or PMA submission, you can search the Product Classification Database.
How does FDA verify premarket submissions?
When a product requires premarket submission, the FDA will verify the declared 510(k) or PMA by comparing the submitted information to CDRH’s data systems. If the 510(k) or PMA information and number is not supplied, or is incomplete or inaccurate, it may delay the review of your entry.
If the information submitted matches the CDRH data system, then compliance is verified; if the information does not match, the FDA may gather additional information or may detain the product. If the product requires a PMA or 510(k) and does not have one, it will be subject to refusal.
How do I obtain information on premarket submissions for specific products?
The FDA maintains public 510(k) and PMA databases. You can search the releasable 510(k) and PMA databases to obtain 510(k) and PMA information for a specific product.
Medical device classification
Currently medical devices fall into one of three medical device classifications: Class I, Class II, and Class III. Class I includes devices with the lowest risk and Class III includes those with the greatest risk. For more information please visit the Classify Your Medical Device page. In some circumstances devices are unclassified. For more information review the Medical Device Classification Product Codes Guidance for Industry and FDA Staff. This document also includes information regarding section 513(g) of the Act, which provides a means to obtain the FDA's view regarding the classification and regulatory requirements that may be applicable to a particular device.
How can I determine the requirements to import a medical device?
The FDA has publicly available databases to determine the classification of a device. This database will help you determine the submission type required for a device.
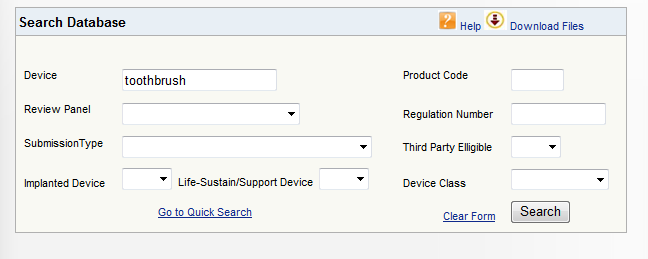
The following is an example of a medical device classification search for a manual toothbrush. Depending on the Class of the medical device you are importing, the submission types will differ.
Step 1: Search
Searching on the word toothbrush will provide a list of device products containing “toothbrush”.
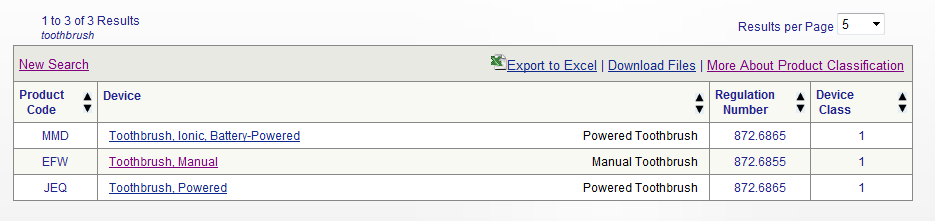
Step 2: View the Results
Clicking on the device of interest, “Toothbrush, Manual” will bring up more information.
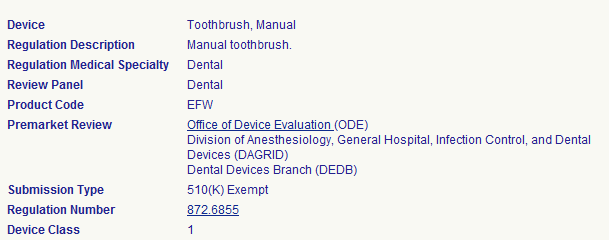
Step 3: View the Details
The device class will be displayed as 1, 2, or 3. In this example, a manual toothbrush is a class 1 medical device. A manual toothbrush is 510(k) exempt, meaning it does not require premarket notification or approval.
Affirmation of Compliance codes for medical devices
Affirmation of Compliance (A of C) codes are three letter codes that can be provided at the time of import to facilitate the FDA review. The FDA uses A of C codes to assist in verifying that your product meets the appropriate requirements. Providing the correct A of C codes reduces the likelihood that your shipment will be held for further the FDA entry review during the FDA’s import screening process. Submission of A of C codes is only mandatory in some instances and is not required for all scenarios. Submitting voluntary A of C codes in addition to all mandatory A of C codes may expedite initial screening and review of your entry. For information on medical device A of C codes as well as descriptions and examples of the medical device affirmation of compliance codes, refer to the “Affirmation Of Compliance References” at the bottom of the affirmation of compliance codes page.
Combination products
Combination products are therapeutic and diagnostic products that combine drugs, medical devices, and/or biological products. Based on primary mode of action, a combination product is assigned to a “lead-center” that has primary jurisdiction for its regulation. For more information on combination products, including product jurisdiction and assignment of the lead center, visit the Frequently Asked Questions About Combination Products page.
Personal importation of medical devices
For more information on the FDA’s Personal Importation Policy visit our Personal Importations page.
Decorative contact lenses
Decorative contact lenses are considered medical devices. The FDA oversees their safety and effectiveness, just like contact lenses that correct your vision. For more information visit our Decorative Contact Lenses page.
Device labeling
For information on labeling a medical device visit our Device Labeling page.
(800) 638-2041
(301) 796-7100
DICE@fda.hhs.gov
Information-Medical Devices/Radiation Products
Division of Industry and Consumer Education
CDRH-Center for Devices and Radiological Health
Food and Drug Administration
10903 New Hampshire Avenue
Silver Spring, MD 20993