Antimicrobial Resistance and Medical Devices
Antimicrobial agents, such as antibiotics, are drugs that work by killing or slowing the growth of targeted microorganisms (bacteria, fungi, viruses, or parasites) responsible for causing infections.
Antimicrobial susceptibility tests (ASTs) are medical devices used in clinical laboratories to determine which antibiotics (or other antimicrobial agents) are likely to be effective against a particular microorganism causing an infection and what dose is needed to be effective.
All AST devices sold in the U.S. for use in clinical laboratories must be reviewed and cleared by the FDA’s Center for Devices and Radiological Health (CDRH) prior to marketing. In addition to conventional ASTs, several rapid diagnostic tests are also available to detect genetic information, or resistance markers, associated with antimicrobial resistant bacteria. These are also reviewed and cleared by CDRH.
On this page:
- Global Impact of Antimicrobial Resistance
- Antimicrobial Susceptibility Testing - Helping Health Care Providers Determine the Correct Antimicrobial Agent for Treatment
- CDRH’s role in antimicrobial susceptibility testing and antimicrobial resistance
- FDA’s Involvement in National Antimicrobial Resistance Activities
- Antimicrobial Resistance and Health Equity
- Related Links
Global Impact of Antimicrobial Resistance
Antimicrobial resistance (AMR) occurs when microorganisms change over time and no longer respond to drugs. According to the Centers for Disease Control and Prevention (CDC), each year in the United States at least 2.8 million antimicrobial-resistant infections occur, and more than 35,000 people die as a result.
The United Nations (UN) considers AMR a global threat and warns that if no action is taken, drug-resistant diseases could cause 10 million deaths each year by 2050. The UN has stated that by 2030, antimicrobial resistance could force up to 24 million people into extreme poverty.
The World Health Organization has outlined priorities related to AMR in their publication, “WHO Strategic Priorities on Antimicrobial Resistance: Preserving antimicrobials for today and tomorrow.”
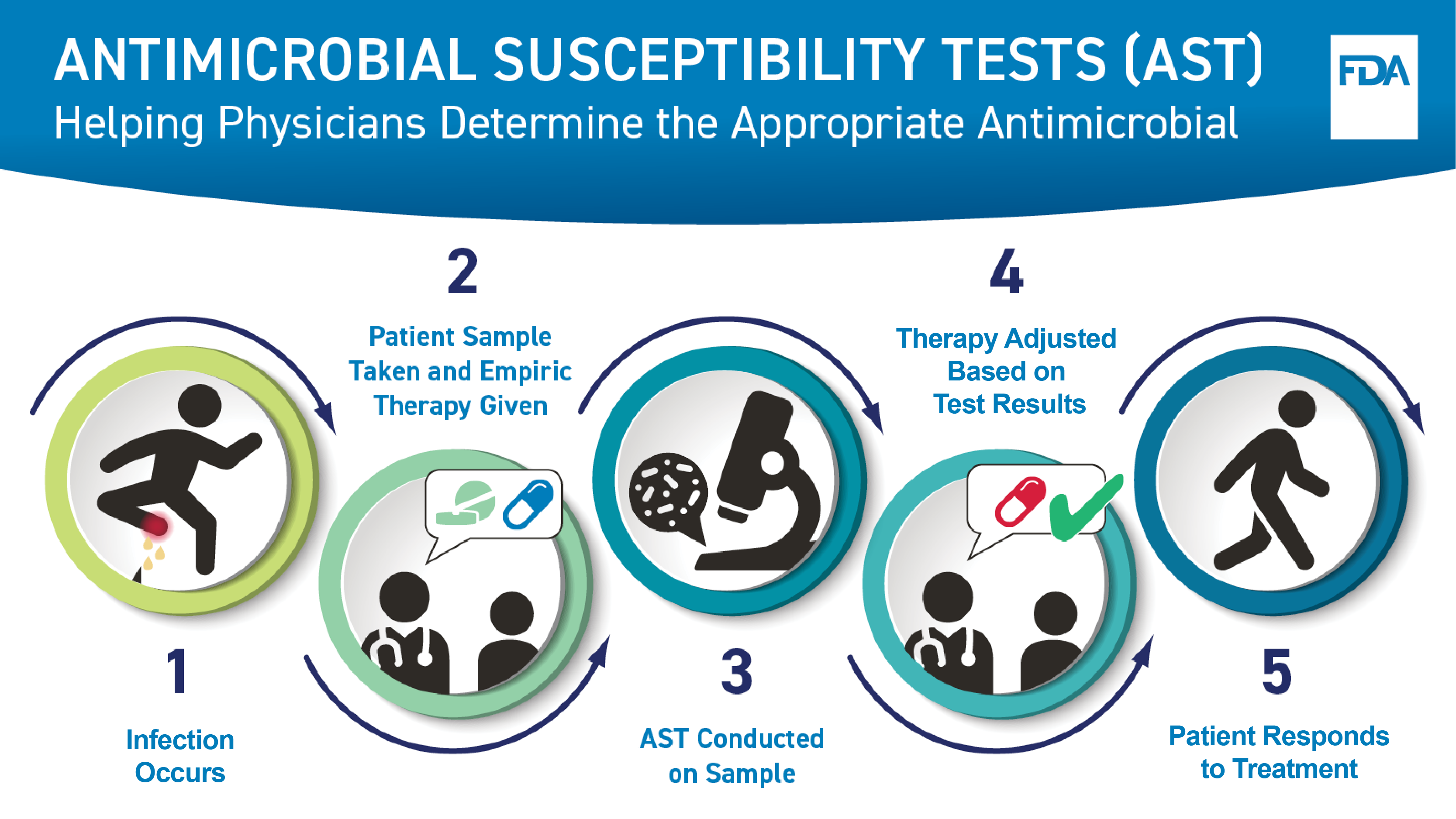
Antimicrobial Susceptibility Testing - Helping Health Care Providers Determine the Appropriate Antimicrobial Agent for Treatment
This graphic shows how an antimicrobial susceptibility test, or AST, helps a health care professional determine the correct drug to treat a bacterial infection. First, an infection occurs. Next, a patient sample is taken for testing and an empiric therapy is provided to the patient. Third, an AST is conducted on the sample. Next, the treatment is adjusted based on the test results, as needed, to ensure the most appropriate therapy, or drug, is given. Last, the patient responds to treatment.
Sample Taken from Patient
When someone has a potential bacterial or fungal infection, the health care provider gives the initial, empiric drug therapy to the patient based on clinical experience until more information can be obtained from testing. The health care provider takes a sample from the site of the infection, such as:
- Urine to evaluate a potential urinary tract infection.
- Blood if a bloodstream infection is suspected.
- Throat swab to diagnose strep throat.
- Swab of a wound for a possible skin infection.
Microorganisms Grown from Sample
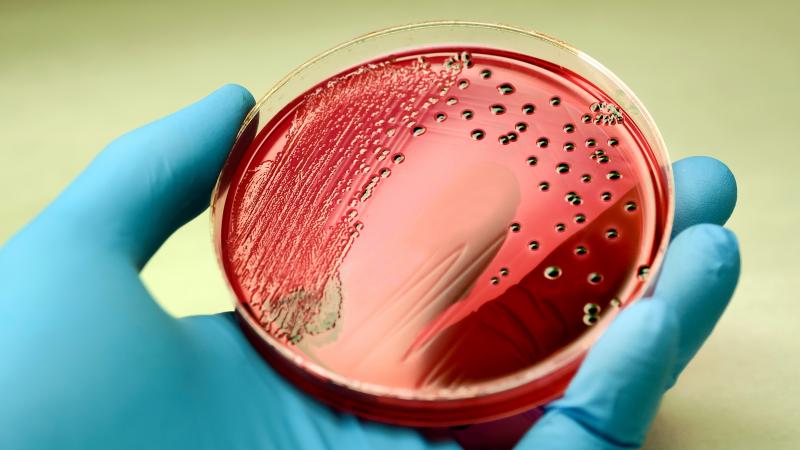
The sample is cultured in a microbiology laboratory. To culture the sample, the laboratory applies the sample to a substance that helps grow microorganisms found in the sample. The laboratory can then identify any organism that grows.
Salmonella bacteria producing red colonies with black center on an agar plate.
Microorganisms Tested with Antimicrobial Agents
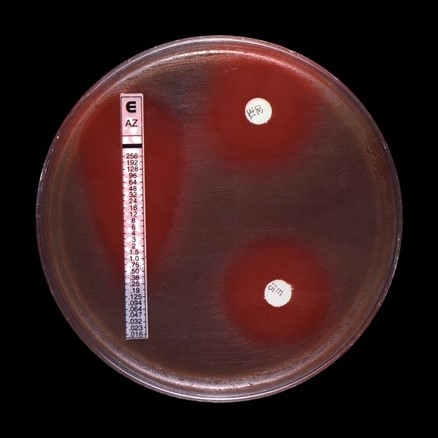
The isolated organisms are then tested with several different types of antimicrobial agents to determine which antimicrobial agents will kill the organism or slow its growth. There are various methods laboratories use to test antimicrobial agents. Some require visual reading of “zones” of inhibition, an area without visible bacteria growth, around a paper disk or plastic strip containing a range of concentrations of the drug. The type of test that uses a long strip is called a gradient diffusion test. It contains multiple concentrations of a drug and determines the lowest drug concentration [known as the minimum inhibitory concentration (MIC)] that will kill the organism or slow its growth. The type of test that shows a halo around a disk, which represents the zone of inhibition, is a disk diffusion test and the size of the halo shows which antibiotics can inhibit the bacteria. Other test methods, such as the broth microdilution method, involve evaluating microbial growth after exposure to a series of drug concentrations included in a plate to determine the MIC.
Results for two types of antibiotic susceptibility tests are shown in this image, a gradient diffusion test and a disk diffusion test to determine the antimicrobial susceptibility of group-B strep.
This image shows a broth microdilution antimicrobial susceptibility test including clindamycin in row A, penicillin in row F and erythromycin in row G.
Tests Interpreted
The ASTs then need to be interpreted to determine which drug will likely be most effective to treat a person based on the organism that caused the infection. Antimicrobial susceptibility test interpretive criteria (STIC), also known as “breakpoints”, are the criteria used to interpret AST results. STIC are updated as microorganisms change over time and are used to identify whether the microorganism is susceptible to a particular drug. The FDA’s Center for Drug Evaluation and Research (CDER) maintains a website with the most up to date STIC for antibacterial and antifungal drugs, including FDA’s recognition of STIC established by standards development organizations (SDOs).
Appropriate Drug Prescribed
The laboratory reports the results of the AST to the health care provider who will prescribe the appropriate drug for the organism that was identified as the cause of the infection. In a hospital setting, infectious disease physicians and specific hospital staff responsible for promoting the appropriate use of antimicrobials may be consulted when deciding the best treatment option.
CDRH’s role in antimicrobial susceptibility testing and antimicrobial resistance
CDRH addresses antimicrobial resistance by ensuring that antimicrobial susceptibility tests on the market provide accurate results for patient care. CDRH also encourages test manufacturers to keep their devices up to date with current breakpoints as resistance to antibiotics develops with expanded use of drugs.
Companies that develop these devices perform extensive validation testing to ensure the device will accurately measure susceptibility or resistance. CDRH reviews the data from the developer’s validation testing and determines if it meets the standards required for the FDA’s clearance. CDRH also ensures that the package insert supplied with the test includes all the necessary information for laboratories to use the test correctly and report accurate results to health care providers. In FDA’s guidance “Antimicrobial Susceptibility Test (AST) System Devices –Updating Breakpoints in Device Labeling,” the FDA describes least burdensome approaches for AST system device manufacturers to update their device labeling with the updated breakpoints listed on the FDA’s STIC Website. The timely adoption of updated breakpoints helps to maintain device safety and effectiveness. While generally updating the STIC could significantly affect the safety and effectiveness of the AST system device and would therefore require a 510(k) submission prior to updating the device labeling, manufacturers that choose to follow the recommendations described in the guidance can incorporate updated breakpoints in their AST system device labeling without a 510(k), if pre-specified criteria are met. FDA anticipates that this guidance will facilitate timely adoption of updated breakpoints in AST system devices. Any questions on this guidance or updating breakpoints should be sent to ASTdevices@fda.hhs.gov.
FDA’s Involvement in National Antimicrobial Resistance Activities
The U.S. has a National Action Plan for Combating Antibiotic-Resistant Bacteria which outlines domestic and international goals to slow the emergence of antimicrobial resistance, prevent the spread of resistant infections, advance the development and use of rapid and innovative diagnostic tests to detect antibiotic resistance, and reduce the number of infections and deaths caused by drug-resistant pathogens. In addition, the Plan supports the development of new antimicrobial agents, other therapeutics, and vaccines.
The CDC leads the U.S. public health response to combat antimicrobial resistance. Funding through their Antimicrobial Resistance (AR) Solutions Initiative supports all 50 state health departments, several local health departments, and Puerto Rico, Guam, and the U.S. Virgin Islands. The FDA works with the CDC to expand and enhance the Antimicrobial Resistance Isolate Bank (AR Isolate Bank), which makes bacterial and yeast isolates available free of charge to requesting institutions as a resource to support the development of drugs and diagnostic tests. Isolates from the AR Isolate Bank may be used by manufacturers in studies to support the FDA premarket notification application of antimicrobial susceptibility tests.
Antimicrobial Resistance and Health Equity
AMR threats cause health disparities across age, race, and gender in settings across health care, the food supply, the environment, and the community. Certain individuals and groups are at increased risk of antimicrobial resistant infections. Risk factors for antimicrobial resistant infections are linked to the social determinants of health—conditions in the places where people live, learn, work, and play—that affect health outcomes and quality of life.
According to the CDC, drug-resistant infections disproportionately impact young children, men who have sex with men (MSM), and groups that have historically experienced greater obstacles based on their racial or ethnic group. Such disparities are also impacted by factors including household income, type of housing (crowding, persons experiencing homelessness), immigration, type of health insurance, access to health care, and education level.
Related Links
Additional Information About AMR
- Antimicrobial Resistance (CDC)
- Antibiotic Prescribing and Use (CDC)
- How Antimicrobial Resistance Happens (CDC) CDC’s Priority to Address Health Equity Issues Across Antibiotic-Resistant Threats
- Health Equity and Antibiotic Resistance (CDC)
- U.S. National Action Plan
- WHO Strategic Priorities on Antimicrobial Resistance: Preserving antimicrobials for today and tomorrow
FDA AMR Programs
- Antimicrobial Resistance Information from FDA
- The CDC & FDA Antibiotic Resistance Isolate Bank
- Immediately-in-effect guidance: Antimicrobial Susceptibility Test System Devices –Updating Breakpoints in Device Labeling