Catheter Controller Recall: Abiomed Removes Automated Impella Controllers due to Pump Driver Circuit Assembly Issues
This recall involves removing certain devices from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it.
Affected Product
Abiomed has issued a letter to affected customers recommending certain Automated Impella Controllers be removed from where they are used or sold:
Product Code(s) | Product Description(s) | UDI-DI(s) |
|---|---|---|
0042-0000-US | Impella Controller, Packaged, US | 00813502010022 |
0042-0000-UK | Impella Controller, Packaged, UK | 00813502011296 |
0042-0000-CA | Impella Controller, Packaged, CA | 00813502011272 |
Full list of affected serial numbers
What to Do
Do not use affected products. Identify and quarantine all affected devices.
On August 20, 2025, Abiomed sent all affected customers a letter recommending the following actions:
- Quarantine and do not use affected products. Contact the Abiomed Field Service team, 1-800-422-8666, option 3 (email ra-abm-fieldaction@its.jnj.com) to initiate the remediation process.
- Forward to anyone in your facility that needs to be informed, such as those who manage, transport, store, stock, or use the subject products.
- If any of the subject products have been forwarded to another facility, contact that facility and provide them with this notice.
Reason for Alert
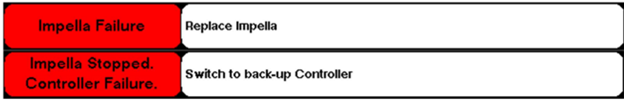
Abiomed stated that certain Automated Impella Controllers have a Pump Driver Circuit Assembly that does not meet current specifications. These Pump Driver Circuit Assemblies contain 25v-rated tantalum capacitors instead of 35v-rated tantalum capacitors which may lead to decreased pump performance or pump stop and trigger an “Impella Failure” or “Impella Stopped. Controller Failure.” alarm (see below image).
In the case of capacitor failure in the Automated Impella Controller, an abrupt pump stoppage or decreased performance of the Automated Impella Controller may occur, potentially resulting in transient hemodynamic instability, loss of circulatory support, or death.
As of August 12, Abiomed has reported one death and no serious injuries associated with this issue.
Device Use
The Automated Impella Controller is the primary user control interface for the Impella Catheters. It controls the Impella Catheter and monitors the catheter for alarms. Impella therapy aims to reduce the work of the heart's left ventricle and provide support for the circulatory system so the heart has time to recover.
Contact Information
Customers in the U.S. with adverse reactions, quality problems, or questions about this recall should contact Abiomed at ra-abm-fieldaction@its.jnj.com.
Additional FDA Resources
- Event in FDA’s Enforcement Report [09/19/2025]
- Entry in CDRH’s Medical Device Recall Database [09/19/2025]
Additional Company Resources
Company-provided information is posted here by the FDA as a public service.
Unique Device Identifier (UDI)
The unique device identifier (UDI) helps identify individual medical devices sold in the United States from manufacturing through distribution to patient use. The UDI allows for more accurate reporting, reviewing, and analyzing of adverse event reports so that devices can be identified, and problems potentially corrected more quickly.
- How do I recognize a UDI on a label?
- AccessGUDID database - Identify Your Medical Device
- Benefits of a UDI System
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program.