UDI Basics
The FDA established the unique device identification system to adequately identify medical devices sold in the United States from manufacturing through distribution to patient use.
Is Your Product a Medical Device?
Determine if your product meets the definition of a device
The Unique Device Identification System final rule (UDI Rule) requires device labelers (typically, the manufacturer) to:
- Include a unique device identifier (UDI) on device labels and packages, except where the rule provides for an exception or alternative.
- If a device is intended for more than one use and intended to be reprocessed before each use, the device labeler must also mark the UDI directly on the device.
- Submit device information to the Global Unique Device Identification Database (GUDID).
- Am I a device labeler?
- Understanding the UDI format
- How do I recognize a UDI on a label?
- Developing a UDI using an FDA-accredited issuing agency’s system
- Meeting compliance dates and requirements
- Understanding exceptions, alternatives, and time extensions
- Submitting information to the GUDID database
- Searching the AccessGUDID database
Am I a device labeler?
A labeler is any person who causes a label to be applied to a device, or who causes the label of a device to be modified, with the intent that the device will be commercially distributed without any subsequent replacement or modification of the label. The addition of the name of, and contact information for, a person who distributes the device, without making any other changes to the label is not a modification for the purposes of determining whether a person is a labeler.
In most instances, the labeler is the device manufacturer, but the labeler may be a specification developer, a single-use device reprocessor, a convenience kit assembler, a repackager, or a relabeler.
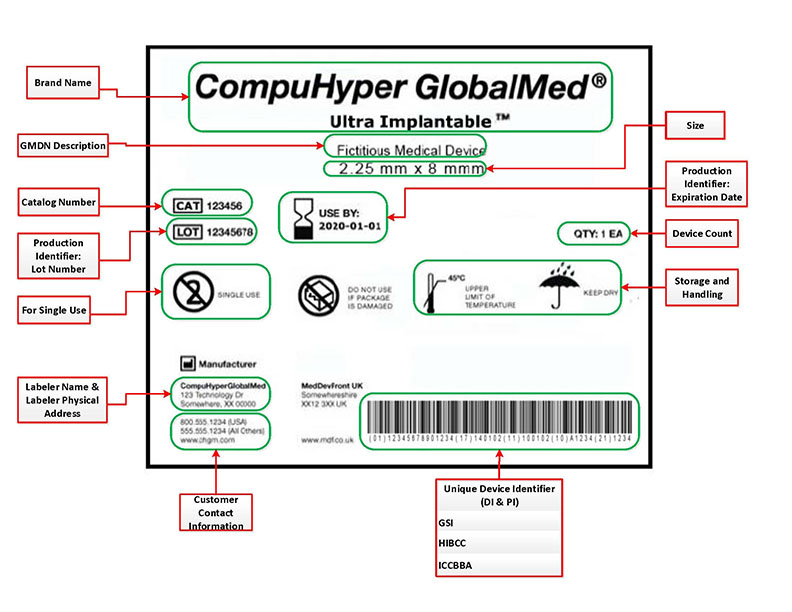
Understanding the UDI Format
A unique device identifier (UDI) is a unique numeric or alphanumeric code that generally consists of the following:
- Device identifier (DI), a mandatory, fixed portion of a UDI that identifies the labeler and the specific version or model of a device.
- Production identifier (PI), a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of a device:
- Lot or batch number within which a device was manufactured
- Serial number of a specific device
- Expiration date of a specific device
- Date a specific device was manufactured;
- Distinct identification code required by §1271.290(c) for a human cell, tissue, or cellular and tissue-based product (HCT/P) regulated as a device.
The device labeler must provide the UDI in two forms on labels and packages:
- Easily readable plain-text
- Machine-readable form that uses automatic identification and data capture (AIDC) technology.
Automatic identification and data capture (AIDC): Any technology that conveys the UDI or the device identifier of a device in a form that can be entered into an electronic patient record or other computer system via an automated process.
In addition, the device labeler must present dates on device labels and packages in a standard format that is consistent with international standards and international practice (YYYY-MM-DD).
How do I recognize a UDI on a label?
The following example of a UDI is for illustrative purposes only
For more information, see UDI Formats by FDA-Accredited Issuing Agency (January 27, 2017).
Developing a UDI Using an FDA-Accredited Issuing Agency’s System
To develop a UDI, device labelers must contact one of the issuing agencies accredited by the FDA. See Contact an FDA-Accredited Issuing Agency for details.
Meeting Compliance Dates and Requirements
The UDI system is being implemented in phases, to ensure a smooth implementation and to spread the costs and burdens of implementation over time, and we are currently in the final phase of implementation which includes the lowest risk devices. See UDI Compliance Policies and UDI Rule Compliance Dates for details on compliance dates and requirements associated with each.
Understanding Exceptions and Alternatives
The UDI Rule outlines certain general exceptions to the UDI requirements (see 21 CFR 801.30, 801.40(d), and 801.45(d)). Additionally, the UDI Rule provides a process for labelers to submit a request for an exception or alternative to a UDI requirement (see 21 CFR 801.55). See UDI Exceptions and Alternatives for details.
Submitting Information to the Global Unique Device Identification Database (GUDID)
Device labelers are required to submit information to the FDA-administered Global Unique Device Identification Database (GUDID). GUDID includes a standard set of basic identifying elements for each device with a UDI and contains ONLY the device identifier (DI), which serves as the key to obtain device information in the database. GUDID does not include the production identifier (PI).
To submit information to GUDID, the device labeler must first request a GUDID account. See Request a GUDID Account for details.
For more information on accessing and using the GUDID database for submitting UDIs, see Global UDI Database (GUDID).
Searching the AccessGUDID Database
Through a partnership with the National Library of Medicine (NLM), the FDA offers AccessGUDID, a separate, searchable database of device identification information available for anyone, including patients, care givers, health care providers, hospitals, and industry.
Using AccessGUDID, you can:
- Search for devices quickly by device name, company, or device identifier (DI)
- Conduct advanced searches using combinations of other fields in the database
- Download GUDID data and export search results.
Note: The AccessGUDID database does not contain information about who uses a device, such as personal privacy information.