Imported Seafood Safety Program
Spotlight
- FDA and Imported Seafood: Ensuring Safety for Everyone (Video) August 2023
Using high-tech analytical tools, innovative food safety programs and border surveillance, the FDA ensures that all imported seafood is held to the same safety standards and regulatory requirements as domestic foods. - Establishing a Regulatory Partnership Arrangement with the FDA (Video) August 2023
A Regulatory Partnership Arrangement allows the FDA to work with foreign country regulators on food safety. There are five steps to creating these partnerships in order to maximize the safety of imported foods for U.S. consumers. - FDA Signs Partnership with Ecuador to Enhance Safety of Shrimp Imports August 2023
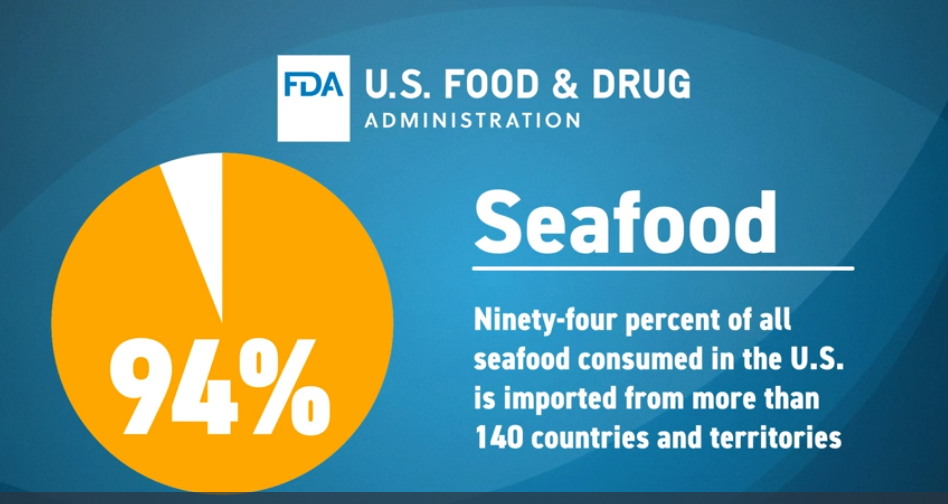
On Aug 25, 2023, the U.S. Food and Drug Administration (FDA) signed a Regulatory Partnership Arrangement (RPA) with Ecuador’s seafood regulatory authority to strengthen food safety in shrimp intended for the U.S. market. The first of its kind, this regulatory partnership serves as an arrangement between the FDA and the Vice Ministry of Aquaculture and Fisheries (VMAF) to work more closely to reinforce food safety practices along the entire supply chain. Shrimp is the most consumed seafood in the United States, the vast majority of which is imported. Ecuador is one of the leading exporters of aquacultured shrimp to the United States. - Enhancing the Safety of Imported Shrimp Through Regulatory Partnerships May 2022
Steven Bloodgood, Acting Director for the Division of Seafood Safety and Fazila Shakir, MHS, Regulatory Cooperation and Partnerships Staff Director, discuss regulatory partnerships to help ensure the safety of imported food.
FDA is responsible for the safety of all fish and fishery products entering the United States. The agency uses every available tool to identify immediate or potential threats as well as the best course of action to protect public health and safety. As part of the FDA’s import safety effort, the agency provides as much available information and guidance as possible to consumers, industry, and government about seafood safety.
- Imported Seafood Safety Goals
- HACCP
- Foreign Inspections and Global Presence
- PREDICT
- Foreign Country Assessments
- Food Safety Modernization Act
- Integrated Food Safety System
- National Residue Monitoring Program
- Consumer Information
Imported Seafood Safety Goals
The FDA Strategy for the Safety of Imported Food (Import Strategy) guided by four goals is the roadmap for the FDA’s comprehensive approach to the safety of imported seafood.
- Food Offered for Import Meets U.S. Food Safety Requirements
- FDA Border Surveillance Prevents Entry of Unsafe Foods
- Rapid and Effective Response to Unsafe Imported Food
- Effective and Efficient Food Import Program
The Activities for the Safety of Imported Seafood details how established FDA regulation and innovative programs and technology are employed to support each of the four goals as they relate to imported seafood safety. These include proactively engaging and establishing partnerships with FDA regulatory counterparts in countries that export seafood to the United States; exploring the use of Artificial Intelligence (AI), specifically Machine Learning (ML), to strengthen predictive analytics; and developing new tools that leverage technology such as geographic information system (GIS) to provide spatial intelligence about potential seafood hazards.
Videos & Recordings
FDA and Imported Seafood: Ensuring Safety for Everyone (Video)
Using high-tech analytical tools, innovative food safety programs and border surveillance, the FDA ensures that all imported seafood is held to the same safety standards and regulatory requirements as domestic foods.
Establishing a Regulatory Partnership Arrangement with the FDA (Video)
A Regulatory Partnership Arrangement allows the FDA to work with foreign country regulators on food safety. There are five steps to creating these partnerships in order to maximize the safety of imported foods for U.S. consumers.
Hazard Analysis and Critical Control Points
FDA’s multifaceted and risk-informed seafood safety program relies on various measures of compliance with its seafood Hazard Analysis and Critical Control Points (HACCP) regulations, which describe a management system in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product.
For imported seafood, these measures include:
- inspections of foreign processing facilities,
- sampling of seafood offered for import into the United States,
- domestic surveillance sampling of imported products,
- inspections of seafood importers,
- evaluations of filers of seafood products,
- foreign country program assessments, and
- relevant information from our foreign partners and FDA overseas offices.
Foreign Inspections and Global Presence
FDA has increased the number of foreign site inspections in recent years and is working globally to better accomplish its domestic mission to promote and protect the public health of the United States. FDA has strengthened and better coordinated its international engagements by establishing permanent FDA posts abroad in strategic locations. The posting of FDA staff in certain overseas regions is a key part of the agency's strategy for expanding oversight of imported food.
PREDICT
FDA is also implementing a new screening system for imports, the Predictive Risk-based Evaluation for Dynamic Import Compliance Targeting (PREDICT), which will improve the current electronic screening system by targeting higher risk products for exam and sampling and minimizing the delays of shipments of lower risk products. PREDICT will improve the agency’s ability to detect trends and investigate patterns. This, in turn, will help to make more efficient use of FDA’s import resources and allow FDA to adjust import sampling levels for seafood products over time and as appropriate.
Foreign Country Assessments
Foreign country assessments are systems reviews that offer FDA a broad view of the ability of the country’s industry and regulatory infrastructure to control aquaculture drugs. These assessments allow FDA to become familiar with the controls that a country’s competent authority is implementing for the distribution, availability, and use of animal drugs. FDA uses country assessments to evaluate the country’s laws for, and implementation of, control of animal drug residues in the aquaculture products it ships to the United States.
The country assessment program helps FDA direct its foreign inspection and border surveillance resources more effectively and efficiently and allows FDA to work directly with countries to resolve drug residue problems.
FDA uses information from country assessments to:
- target (i.e., increase or decrease) surveillance sampling of imported aquaculture products;
- inform its decisions on what new analytical methods it needs to develop and what drugs or chemicals it should target for surveillance sampling;
- inform its planning of foreign seafood HACCP inspections;
- provide additional evidence for potential regulatory actions, such as an import alert;
- improve collaboration with foreign government and industry contacts to achieve better compliance with FDA’s regulatory requirements; and
- understand the causes for significant changes in a country’s drug residue problems, such as a sudden spike in noncompliant samples.
Results of Country Assessments
- The assessment trip to China in 2006 was a key consideration in issuance of the China country-wide import alert for specific aquaculture products from China in 2007.
- The country assessments for China in 2006, Chile in 2008, and India in 2010 were considered and resulted in increased sampling and testing under the compliance program and special assignments for aquaculture products from these countries (e.g., eel from China, salmon from Chile, and shrimp from India).
Food Safety Modernization Act of 2011
FDA conducts its seafood safety oversight activities in conformance with its statutory authorities, which have recently been expanded by the Food Safety Modernization Act (FSMA). FSMA represents the first major overhaul of FDA’s food safety law in more than 70 years and will transform FDA’s food safety program. FSMA closes significant and longstanding gaps in FDA’s food safety authority, with new safeguards to prevent, rather than react, to food safety problems, and gives FDA important new tools to ensure that imported seafood is as safe as domestic seafood.
Integrated Food Safety System
FDA collaborates with the President’s Food Safety Working Group to modernize food safety by building collaborative partnerships with consumers, industry and regulatory partners.
For example, FDA and the National Marine Fisheries Service's (NMFS) Seafood Inspection Program have certain common and related objectives in carrying out their respective regulatory and service activities that lend themselves to cooperation under a Memorandum of Understanding (MOU) that sets forth the working arrangements between the agencies that facilitate each agency’s efforts to discharge its responsibilities related to the inspection of fish and fishery products.
National Residue Monitoring Program
In addition to implementing the new FSMA authorities, FDA will continue the national residue monitoring program and recognizes the benefit of such a program to ensure that foods are not contaminated with illegal animal drug residues. FSMA directs FDA to establish a program for testing of food by accredited laboratories and will require that food be tested by accredited laboratories in some circumstances, such as in support of admission of imported food. FDA is developing the laboratory accreditation program as part of its FSMA implementation efforts.
Consumer Information
The government-wide FoodSafety.gov web site provides a widget that displays the latest recalls and food safety alerts from both FDA and USDA. If you have a mobile device, such as an Android phone, you may be able to download an application to get recalls direct to your phone by going to Recalls.gov.