What are Medical Countermeasures?
Medical countermeasures, or MCMs, are FDA-regulated products (biologics, drugs, devices) that may be used in the event of a potential public health emergency
About MCMs | FDA's role | Emergency use | Animal Rule | Infographic
About MCMs
Medical countermeasures, or MCMs, are FDA-regulated products (biologics, drugs, devices) that may be used in the event of a potential public health emergency stemming from a terrorist attack with a biological, chemical, or radiological/nuclear material, or a naturally occurring emerging disease.
MCMs can be used to diagnose, prevent, protect from, or treat conditions associated with chemical, biological, radiological, or nuclear (CBRN) threats, or emerging infectious diseases.
MCMs can include:
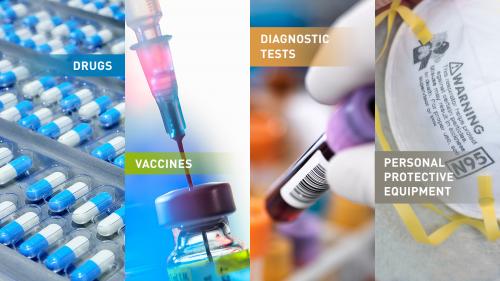
- Biologic products, such as vaccines, blood products and antibodies
- Drugs, such as antimicrobial or antiviral drugs
- Devices, including diagnostic tests to identify threat agents, and personal protective equipment (PPE), such as gloves, respirators (certain face masks), and ventilators
What is the FDA’s role in supporting the development of medical countermeasures?
Through our medical countermeasure (MCM) regulatory activities, the FDA works with partners at all levels of government—local, state, national and international—to support MCM-related public health preparedness and response efforts. The FDA is part of the U.S. Department of Health and Human Services (HHS) Public Health Emergency Medical Countermeasures Enterprise (PHEMCE), which coordinates MCM-related efforts across HHS and USG interagency partners.
The FDA also works with non-government organizations, universities and research centers, and industry to further the development of MCMs for public health emergency preparedness.
How are MCMs accessed and used in emergencies?
Depending on the emergency and public health need, during a public health emergency, MCMs may be provided by the Strategic National Stockpile (SNS), which is overseen by the Administration for Strategic Preparedness and Response (ASPR), or through state and local stockpiles or other pharmaceutical caches. MCMs are usually dispensed or administered by health care workers and public health responders under official federal, state, and/or local emergency response plans.
In some cases, at the time of a public health emergency, MCMs may be approved by the FDA and will be used in approved ways during a response. Some MCMs may not be approved yet, or they may be approved but not for the indication under consideration during the emergency.
Because of its role in regulating medical products, and the nature of some of these products, the FDA may need to use special authorities to allow the use of such MCMs in impacted populations during or in anticipation of emergencies. Mechanisms the FDA can use to allow the emergency use of MCMs include the Emergency Use Authorization (EUA) authority and several authorities related to the emergency use of approved MCMs.
What is the Animal Rule?
Before a medical product can be approved by the FDA, the sponsor must demonstrate efficacy—that the product works. In some cases, such as developing MCMs for potential bioterror threats, human challenge studies (exposing people to the threat agent) would not be ethical or feasible.
In these cases, the FDA may grant approval based on well-controlled animal studies, when the results of those studies establish that the drug or biologic product is reasonably likely to produce clinical benefit in humans. The product sponsor must still demonstrate the product’s safety in humans. Visit the FDA Animal Rule information page for more.
Still have questions? Email AskMCMi@fda.hhs.gov.
Infographic
Descriptive text for the What are medical countermeasures? infographic
Medical countermeasures, or MCMs, are FDA-regulated products that may be used in a public health emergency stemming from a terrorist attack with or accidental release of a biological, chemical, or radiological/nuclear agent, or a naturally occurring emerging infectious disease.
MCMs prevent, protect against, treat, or diagnose diseases or health effects caused by CBRN threat agents (CBRN = chemical, biological, radiological, nuclear), and emerging infectious diseases.
Examples of MCMs include biologic products (vaccines, blood products, antibodies), drugs (antimicrobials, chemical threat antidotes, treatments for radiation injury), and devices (diagnostic tests and personal protective equipment, or PPE, which includes gloves, respirators/certain masks, and gowns.
Some examples of FDA’s MCM roles:
FDA is responsible for assessing safety and effectiveness of MCMs for FDA approval. Activities include: review evidence for approval, regulatory science, policy and legal support, and professional development.
FDA works with partners to advance development and availability of MCMs. Partners include: governments (state, local, territorial, tribal, national, and international), international organizations, medical and scientific community, industry, and the Public Health Emergency Medical Countermeasures Enterprise (PHEMCE), which is comprised of federal agencies.
To prepare for and respond to emerging threats, FDA can issue Emergency Use Authorizations (EUAs) to enable access to MCMs prior to approval, or for unapproved uses. View the latest MCMi Program Update for the latest MCM approvals, EUA information, and much more. FDA also has other legal authorities to facilitate emergency access to MCMs.
FDA Medical Countermeasures Initiative (MCMi):
MCMi is an FDA-wide initiative across FDA product centers (including CBER, CDER, and CDRH) and offices to coordinate MCM development, preparedness, and response.
Questions? Learn more at www.fda.gov/medicalcountermeasures, or email us at AskMCMi@fda.hhs.gov.
Updated June 2024.


