Advancing Real-World Evidence Program
On this page:
- Goals
- Eligibility Criteria
- Selection for Participation
- Submission Deadlines and Process
- Content and Format of the Initial Meeting Request
- Disclosure Agreement
- Content and Format of Follow-up Meeting Requests
- Advancing RWE Program Timeline
- Contact Us
- Frequently Asked Questions
- Learn more about Real-World Evidence
As announced in the Federal Register notice published on October 20, 2022, FDA is conducting an Advancing Real-World Evidence (RWE) Program, which seeks to improve the quality and acceptability of RWE-based approaches in support of new intended labeling claims, including approval of new indications of approved medical products, or to satisfy post-approval study requirements. The Advancing RWE Program fulfills an FDA commitment under PDUFA VII, incorporated as part of the FDA User Fee Reauthorization Act of 2022.
The Advancing RWE Program provides sponsors who are selected into the Program the opportunity to meet with Agency staff—before protocol development or study initiation—to discuss the use of RWE in medical product development. The Advancing RWE Program is an optional pathway for sponsors submitting RWE proposals; established procedures to engage with the Agency will continue to be available.
Meetings will be conducted by FDA’s Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) during fiscal years 2023 to 2027. Oncology applications will include participation from the Oncology Center of Excellence. To promote awareness of characteristics of RWE that can support regulatory decisions, study designs discussed through the program may be presented by FDA in a public forum (e.g., in a guidance or public workshop).
Goals of the Advancing Real-World Evidence Program
The Advancing RWE Program is designed to:
- identify approaches for generating RWE that meet regulatory requirements in support of labeling for effectiveness (e.g., new indications, populations, dosing information) or for meeting post-approval study requirements;
- develop agency processes that promote consistent decision-making and shared learning regarding RWE; and
- promote awareness of characteristics of RWE that can support regulatory decisions by allowing FDA to discuss study designs considered in the Advancing RWE Program in a public forum.
Eligibility Criteria
- The sponsor has an Investigational New Drug (IND) or pre-IND number for the medical product in the Advancing RWE Program meeting request (to obtain a pre-assigned number, see CDER instructions or CBER SOPP 8117).
- The proposed RWE is intended to meet regulatory requirements in support of labeling for effectiveness (e.g., new indications, populations, dosing information) or for meeting post-approval study requirements.
- The sponsor and FDA reach agreement on the study design information to be publicly disclosed.
Selection for Participation
FDA welcomes submissions related to any eligible RWE proposal. Given the limited number of requests accepted per submission cycle, however, FDA will select requests based on their potential regarding fit-for-use data, adequate study design, and appropriate regulatory conduct. Consideration will also be given to promoting diversity of data sources, study designs, analytical methodologies, and regulatory indications, as well as to diversity of diseases under study and FDA Centers and Offices involved.
Submission Deadlines and Process
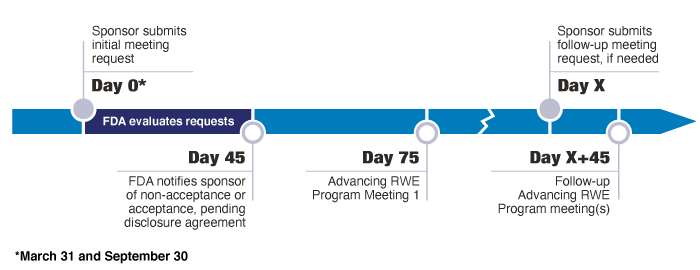
The semi-annual submission deadlines for initial meeting requests to the Advancing RWE Program are March 31 and September 30. Sponsors may submit an initial meeting request to the Advancing RWE program on a rolling basis through March 31, 2027. FDA will review all meeting requests received in the preceding 6-month submission cycle after each submission deadline.
| Advancing RWE Program Semi-annual meeting request submission deadlines | |
|---|---|
| March 31 | Sept. 30 |
FDA will accept one to two primary meeting requests and up to two alternates per submission cycle in FY 2023-2024, and one to four primary meeting requests and up to four alternates per submission cycle in FY 2025-2027. Sponsors will be notified whether they will proceed to discussion of disclosures or their meeting has been denied approximately 45 days after the submission deadline. For each meeting request granted as part of the Program, FDA will conduct an initial meeting and, if requested, up to three follow-up meetings.
Meeting requests should be submitted electronically to the relevant application (i.e., pre-IND, IND) with “Advancing RWE Program Meeting Request for CDER” (CDER applications) or “Advancing RWE Program Meeting Request for CBER” (CBER applications) in the subject line of the cover letter. Please review the information about providing regulatory submissions in electronic format. In addition, please notify CDER-RWE@fda.hhs.gov that your Advancing RWE Program meeting request has been submitted to the relevant CDER or CBER application.
Content and Format of the Initial Meeting Request
The information below should be included in the initial request for a meeting under the Advancing RWE Program. For requests that are granted, information provided in the initial meeting request will serve as the basis for discussion at the first meeting. The scope of the requested information is aligned with the goal of soliciting proposals involving real-world data (RWD) before the sponsor makes final decisions on study design.
Proposals should not exceed 12 pages, with no more than two pages for items #1-8 and ten pages for items #9-12. Some of the topics in items #9-12 below may not be pertinent and others not mentioned may be pertinent. Sponsors should decide what information is most relevant to decisions regarding study design, data sources, analyses, and study conduct. Items #9-12 should include discussion of potential strengths and limitations.
Submissions to the Advancing RWE Program that do not adhere to these format and content requirements will not be considered further.
- Product name
- Pre-IND or IND number
- Proposed purpose of the study (e.g., for new labeling claim or to meet post-approval study requirement)
- Proposed indication, as applicable
- Brief history of product development
- Rationale for a real-world evidence approach
- Brief overview of proposed study design
- Elements of the study design that the sponsor considers non-disclosable, if applicable, along with a rationale for exclusion
- Attributes of study design: objectives; design architecture (e.g., randomized trial with pragmatic elements, externally controlled trial, observational cohort study) with a schematic representation; eligibility criteria; covariates of interest; primary and key secondary endpoints; treatment of interest, comparator, and concomitant therapies
- Potential data sources: category (e.g., electronic health records, medical claims, registries, and/or other) and description; data reliability and relevance; validation, timing, and completeness of key data elements; linkage to other data sources; additional data collection.
- Anticipated analysis plan: approximate sample size; analytic plan for primary and key secondary endpoints; approach to confounding factors; definition of follow-up period; handling of intercurrent events, missing or misclassified data, and multiplicity.
- Miscellaneous considerations: pre-specification of study design and conduct; availability and FDA access to patient-level data; approach to human subject protection.
Disclosure Agreement
Within 45 days after the submission deadline, FDA will review the meeting requests, select primary and alternate requests to proceed to disclosure discussions, and notify sponsors of their status.
To promote innovation and to provide better clarity on the acceptability of different types of data sources and study designs, key design elements developed through the Advancing RWE Program may be presented by FDA (e.g., in a guidance or public workshop) as case studies, including while the drug studied has not yet been approved by FDA for the proposed indication or the post-marketing study has not been completed. FDA intends to focus on those elements relevant to the understanding of the RWE and its potential regulatory use. When feasible, FDA will notify a sponsor in advance when the sponsor’s program is the planned focus of a public discussion.
Before FDA grants the initial meeting under the Advancing RWE Program, the Agency and the sponsor must agree on the information that FDA may disclose publicly. In a disclosure agreement with sponsors, FDA intends to include, as applicable, the following categories of information describing data sources and study design:
- Proposed use of RWE
- Support labeling changes for an approved product: new indication; expand use to a new population; change in dose, dose regimen, or route of administration; other labeling change
- Support or satisfy a post-approval study requirement
- Disease/condition of interest
- Real-world data sources
- Category (e.g., electronic health records, medical claims, registries, and/or other) and brief description of data sources
- Data reliability, including data accrual and assurance processes
- Relevance of data to the research question being addressed
- Timing and completeness of key data elements
- Assurance of data integrity
- Validation efforts related to key data elements
- Linkage to other data sources and additional data collection
- Mechanism for FDA access to patient-level data and source records
- Study design characteristics:
- Type of design (e.g., randomized trial that incorporates RWD, externally controlled trial that incorporates RWD, observational cohort)
- Study schema, including exposure and follow-up time
- Sample size and general description of source population
- Sample size and general description of study population
- Choice of control/comparator
- Study endpoints
- Estimand(s) of interest
- Plans for study monitoring
- Randomization and blinding, as applicable
- Analysis plan:
- Null and alternative hypotheses
- Approach to assessing and controlling for bias
- Statistical test(s) for primary and key secondary study endpoints
- Important subgroup analyses
- Approach to handling of missing or misclassified data
- Approach to addressing multiplicity
- Any modifications or amendments to any of the above that occur during interactions about the proposed RWE between submitter and FDA in the context of the Advancing RWE Program.
Excluded from Disclosure:
The Agency does not intend to share specifics regarding the sponsor’s name, treatment of interest (i.e., product name or molecular structure), a complete description of study eligibility criteria, or patient-level data.
It is important that the disclosure aspect of this program be consistently applied to all participating parties. If sponsors believe their program has unique disclosure considerations, they should identify information they consider non-disclosable and provide a rationale for withholding the information. Participation in the Advancing RWE Program, including any agreement on information disclosure, will be voluntary and at the discretion of the sponsor. Sponsors that do not wish to make such disclosures may seek regulatory input through existing channels.
Content and Format of Follow-up Meeting Requests
Sponsors whose initial meeting requests are granted as part of the Advancing RWE Program and who would like to request one of up to three total follow-up meetings should submit a follow-up meeting request and meeting background package electronically. Include “Advancing RWE Program Follow-up Meeting Request for CDER” (CDER applications) or “Advancing RWE Program Follow-up Meeting Request for CBER” (CBER applications) in the subject line of the cover letter. In addition, please also notify CDER-RWE@fda.hhs.gov that your Advancing RWE Program follow-up meeting request and meeting background package have been submitted to the relevant CDER or CBER application.
The follow-up meeting request and meeting background package should include the following information:
- Product name
- Pre-IND or IND number
- Major changes to the proposal, new information, or decisions made since the preceding meeting
- List of questions for discussion with FDA at the follow-up meeting with an explanation of the need or context for the question
A meeting summary will be sent to the requester within 30 days of each initial and follow-up Advancing RWE Program meeting.
Advancing RWE Program Timeline
Frequently Asked Questions
Visit Advancing RWE Program Frequently Asked Questions for more information about the program.
Contact Us
For more information regarding a submission to CDER or CBER, please email CDER-RWE@fda.hhs.gov and include the subject line “Advancing RWE Program.”