Information on Pediatric Medical Cribs and Medical Bassinets Used in Homes, Child Care Settings and Traditional Health Care Facilities
Consumer Product Safety Commission (CPSC) crib standards banning drop-side rail designs for consumer cribs took effect on June 28, 2011 for consumer crib manufacturers and retailers, and on December 28, 2012 for child care centers, family child care homes, and places of public accommodation (such as hotels). Current FDA regulation allows pediatric medical cribs used in health care settings to keep the drop-side rail feature because it is critical for providing appropriate medical care to sick children. The FDA realizes that in certain, uncommon situations, pediatric medical cribs with drop-side rails may need to be used outside of a health care setting.
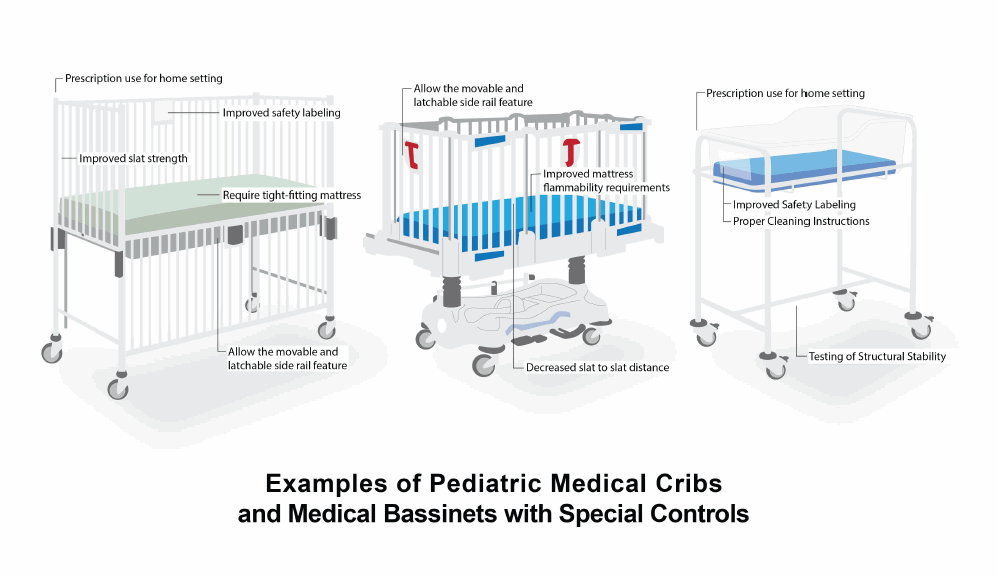
The FDA issued a proposed rule on October 8, 2015, proposing new safety requirements for medical cribs and bassinets used in the treatment and care of pediatric patients which would allow them to be used outside of health care settings when prescribed by a physician. The FDA published the final rule on December 19, 2016, with special controls that establish new safety requirements for pediatric medical cribs and medical bassinets. These include standards for the spacing between the slats in medical cribs, and mattress flammability for medical cribs. The FDA believes that these requirements are necessary to provide reasonable assurance that pediatric medical cribs and medical bassinets are safe and effective.
The final rule will:
- provide continued access by prescription use to pediatric medical cribs with drop-side rails and medical bassinets in a home, child care or other facility when it is medically necessary;
- further reduce potential risks associated with pediatric medical cribs and medical bassinets, such as entrapment or other injuries to the pediatric patient; and
- align applicable safety requirements for pediatric medical cribs with those of cribs for non-medical uses; and
- provide manufacturers with clarity about FDA's safety expectations and requirements by providing more specific design requirements for pediatric medical cribs and medical bassinets.
The FDA is aware that some child care facilities and family child care homes already have one or more pediatric medical cribs in their facility. We encourage child care facilities with questions about pediatric medical cribs to contact their local or state licensing agencies to find out how the CPSC's final rule affects them.
The final rule will be effective 30 days after the publication date in the Federal Register.