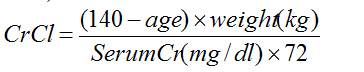FDA Drug Safety Communication: Seizure risk for multiple sclerosis patients who take Ampyra (dalfampridine)
Safety Announcement
Additional Information for Patients
Additional Information for Health Care Professionals
Data Summary
Reference
[7-23-2012] The U.S. Food and Drug Administration (FDA) is updating health care professionals and the public about the risk of seizures in patients with multiple sclerosis (MS) who are starting Ampyra (dalfampridine). Using information received from post-market adverse event reports, FDA recently evaluated seizure risk in MS patients taking Ampyra (dalfampridine). The majority of seizures happened within days to weeks after starting the recommended dose and occurred in patients having no history of seizures (see Data Summary).
|
Facts about (Ampyra) dalfampridine |
|
In addition, FDA is updating the Ampyra drug label to clarify recommendations that kidney function should be checked in patients before starting Ampyra and monitored at least annually while Ampyra treatment continues. Additionally, patients who miss a dose should not take extra doses—an extra dose of Ampyra can increase seizure risk.
Seizures are a known side effect of Ampyra, and seizure risk increases with higher blood levels of the drug. Ampyra is eliminated from the body through the kidneys, and patients with kidney impairment may develop higher blood levels of the drug, thereby increasing their seizure risk. Ampyra should not be used in patients with a history of seizures or who have moderate to severe renal (kidney) impairment (measured as creatinine clearance [CrCl] less than or equal to 50 mL/min).
In patients with mild renal impairment (CrCl 51-80 mL/min), the blood levels of Ampyra may reach levels associated with increased seizure risk. Therefore for patients with mild renal impairment, the use of Ampyra requires careful consideration of the potential benefits of treatment as well as the potential risk of seizure.
FDA reminds health care professionals that there are age-related decreases in renal function, and mild renal impairment is common after age 50, even when serum creatinine is normal. Renal function should be assessed by estimating creatinine clearance (see Data Summary).
Additional Information for Patients
- Ampyra can cause seizures, even if you have never had a seizure before.
- Stop taking Ampyra and call your doctor right away if you have a seizure.
- The chance of having a seizure is higher if you take too much Ampyra or if your kidneys have decreased function. Loss of some kidney function is common after age 50.
- Tell your health care professional if you have kidney problems.
- Your health care professional should order blood tests periodically to evaluate your kidney function.
- Do not take Ampyra if you have ever had a seizure.
- Read the Medication Guide that comes with your Ampyra prescription.
- Ampyra tablets should be taken whole and not divided, crushed, chewed, or dissolved.
- Do not take double or extra doses of Ampyra if a dose is missed. Side effects, including seizures, are more frequent at higher doses.
- Discuss any questions you have about Ampyra with your health care professional.
- Report any side effects you experience to the FDA MedWatch program using the information in the “Contact FDA” box at the bottom of the page.
Additional Information for Health Care Professionals
- Ampyra is contraindicated in patients with a history of seizures or with moderate to severe renal impairment (CrCl 50 mL/min).
- Mild renal impairment is common after age 50.
- The potential benefits of Ampyra treatment should be carefully considered against the risk of seizures before using Ampyra in patients with mild renal impairment (CrCl 51-80 mL/min).
- Most of the seizures reported with Ampyra treatment occurred in patients without a history of seizures.
- A patient’s CrCl (calculated using the Cockroft-Gault equation) should be known before initiating Ampyra treatment and monitored at least annually while Ampyra treatment continues, even when serum creatinine levels appear to be normal.
- The maximum recommended dose of Ampyra is 10 mg twice daily (taken 12 hours apart). Ampyra tablets should be taken whole and not divided, crushed, chewed, or dissolved.
- Tell patients they should not take double or extra doses of Ampyra if a dose is missed. Adverse effects, including seizures, are more frequent at higher doses.
- Ampyra should be discontinued permanently if a seizure occurs.
- Report adverse events involving Ampyra to the FDA MedWatch program, using the information in the “Contact FDA” box at the bottom of the page.
Seizures are a known side effect of Ampyra, and seizure risk increases with higher blood levels of the drug. Using information from FDA’s Adverse Event Reporting System (AERS), FDA further evaluated the risk of seizures in MS patients taking Ampyra (dalfampridine). FDA’s analysis identified postmarketing case reports of seizures associated with Ampyra at the labeled recommended dose, with many cases of seizures occurring within the first week of starting Ampyra. The vast majority of seizures occurred in patients without a prior history of seizures. Some patients had been taking other drugs that could have increased the risk of seizures or lowered the seizure threshold. Potentially, age-related renal dysfunction and resultant increases in Ampyra plasma concentrations contributed to the risk of seizure.
Mild renal impairment is common after age 50, even when serum creatinine levels are in the normal range. FDA noted that most patients who experienced a seizure were at least 50 years old and were at risk for mild age-related renal impairment. In patients with mild renal impairment (CrCl 51-80 mL/min), the blood levels of Ampyra may reach levels that have been associated with an increased risk of seizures. The potential benefits of Ampyra treatment must therefore be carefully considered against the potential risk of seizures before using Ampyra in patients with mild renal impairment.
Before starting treatment with Ampyra, renal function should be assessed; if CrCl is unknown, it can be estimated using the Cockcroft-Gault equation (multiplied by 0.85 for women):
Patients with a creatinine clearance between 51-80 mL/min are considered to have mild renal impairment and are at a greater risk of seizure when taking Ampyra. Use of Ampyra remains contraindicated in patients with a creatinine clearance ≤ 50 mL/min.
- Acorda Therapeutics, Response to FDA's Information Request dated July 11, 2012, Submitted on July 11, 2012.
Related Information
- Ampyra (dalfampridine) Information
- FDA Drug Safety Podcast for Healthcare Professionals: Seizure risk for multiple sclerosis patients who take Ampyra (dalfampridine)
- Comunicado de la FDA sobre la seguridad de los medicamentos: Riesgo de convulsiones en pacientes con esclerosis múltiple que toman Ampyra (dalfampridine)