Using Fat-Dissolving Injections That Are Not FDA Approved Can Be Harmful
How does a fat-dissolving injection (injection lipolysis) work?
Injection lipolysis (“lipo” = fat, “lysis” = breakdown) is a nonsurgical procedure that involves administering a series of injections under the skin to break down fat cells in the areas around the injection sites. FDA has received reports of consumers who have used fat-dissolving injections that are not FDA approved and that have caused adverse reactions (bad side effects).
Adverse reactions from using unapproved fat-dissolving injections
Fat-dissolving injections that are not FDA approved are being marketed and sold online under brand names such as Aqualyx, Lipodissolve, Lipo Lab, Kabelline, and others. Sellers of fat-dissolving injections have claimed their products reduce fat deposits in areas of the body such as the chin, back, thighs, upper arms, and stomach. Common ingredients in these injections include phosphatidylcholine (PPC) and sodium deoxycholate (DC). These ingredients have been used alone or together, sometimes referred to as “PCDC injections.” These ingredients pose a significant safety risk because they are unapproved, which means the FDA has not evaluated their safety or effectiveness.
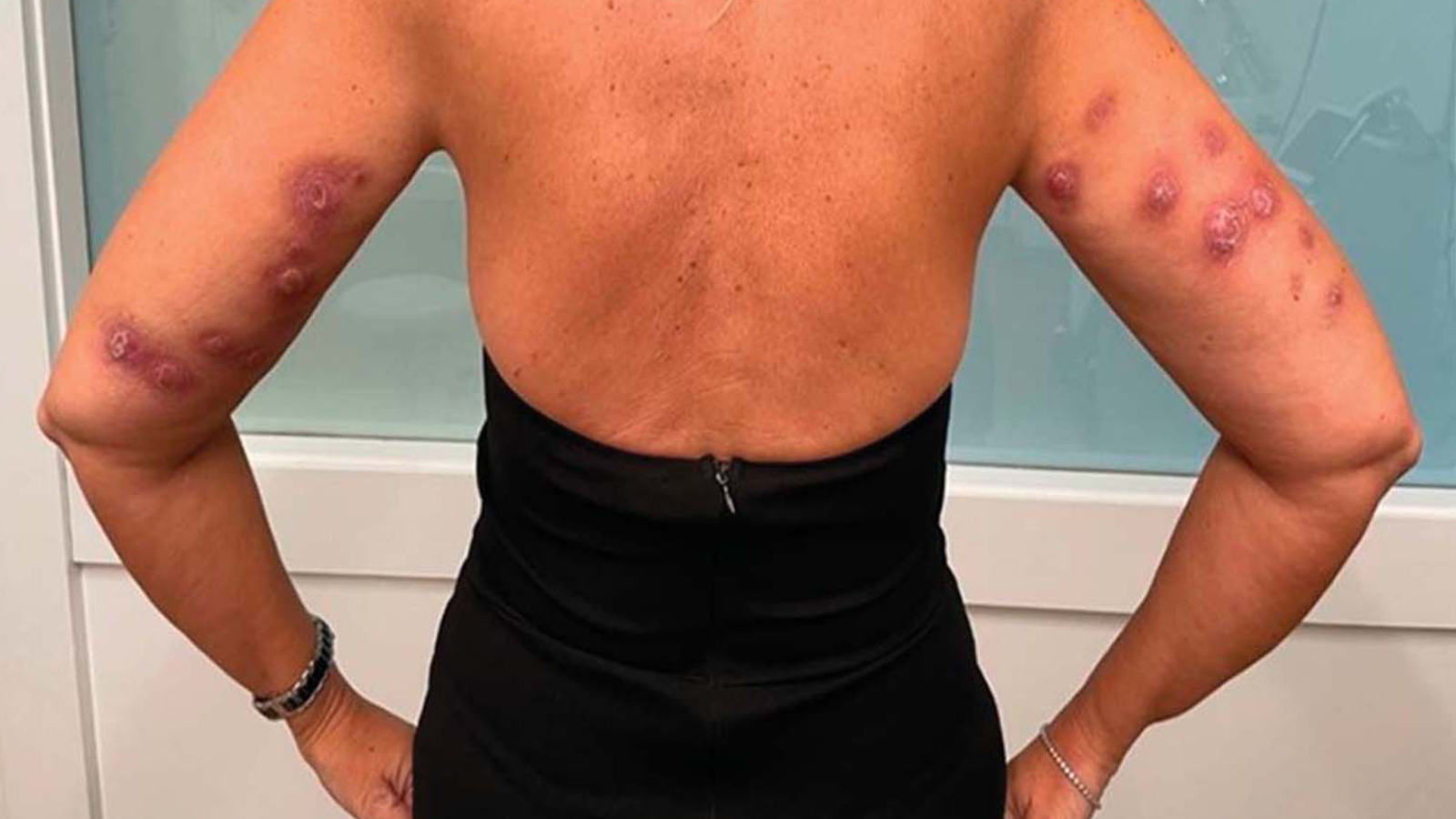
FDA has received reports about consumers who experienced adverse reactions such as permanent scars, serious infections, skin deformities, cysts, and deep, painful knots after receiving unapproved fat-dissolving injections. Some consumers received the injections at clinics or med spas by personnel who might not have been properly licensed to give the injections. In addition, some consumers who reported adverse reactions purchased the unapproved fat-dissolving drugs online and injected the drugs themselves. Figures 1 and 2 show an example of a woman who developed infected knots on both arms one week after receiving unapproved fat-dissolving injections.
Figure 1: Multiple infected knots at the injection sites on the upper arm of a woman who received injections with Lipodissolve, a drug that is not FDA approved, and sought treatment at a dermatology clinic one week after the injections | Permission Credit
Figure 2: Appearance of the knots after two months of antibiotic treatment | Permission Credit
Risks from improper injection techniques
In addition to the risks that come with using unapproved fat-dissolving injections, improper or unsafe injection practices by unlicensed personnel could increase the risk for scarring and skin infections and result in serious complications. Safe and effective use of these products depend on the correct number and location of injections, proper needle placement, and proper administration technique. Consumers should not purchase ingredients for unapproved fat-dissolving injections or inject the drugs themselves.
Has FDA approved any fat-dissolving injections?
A prescription drug called Kybella, the brand name for deoxycholic acid, is the only fat-dissolving injectable drug that is FDA approved to reduce the amount of fat under the chin, or “double chin,” in adults. FDA approval means that the agency has evaluated the drug for quality, safety and effectiveness. FDA has only evaluated this drug for use under the chin; it is not approved for use in any other areas of the body.
The FDA-approved label for Kybella notes that injections should be administered only by a health care professional. Please refer to the FDA-approved Kybella label for a full list of the side effects associated with this drug.
What do I need to know before receiving fat-dissolving injections?
- Consult a health care professional to receive the FDA-approved injection.
- Do not purchase fat-dissolving products from websites and attempt to inject them yourself. Not only might the product be ineffective, you might experience serious complications such as infections or permanent scars.
- If you have received or are thinking about receiving these injections, tell your regular health care provider.
- If you have received these injections and are experiencing side effects, tell your health care provider. If you do not have a regular health care provider, seek medical care.
How do I report side effects to FDA?
In addition to seeking medical care for any adverse reactions after receiving fat-dissolving injections, we urge you to report them, as well as adverse reactions from any other medications, to FDA’s MedWatch program. The MedWatch program helps FDA track medication safety issues:
- Complete and submit the report online; or
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.
Permission Credit
Photo used with permission | Kaur, H, C Reyes-Barron, WH Sipprell, A Cameron, T Louie, PR Tsai, and G Scott, 2022, Painful Purulent Nodules Caused by Mycobacterium at Site of Lipodissolve Injections, Am J Dermatopathol, 44(4):257–259.