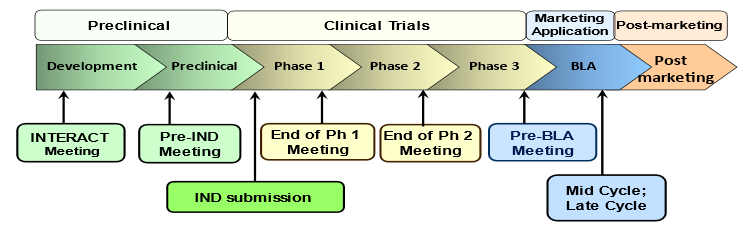
Interactions with Office of Therapeutic Products
The Office of Therapeutic Products, known as OTP, is one of three product offices within CBER responsible for regulatory oversight of biological products. OTP’s mission is to promote public health through a data-driven process to provide regulatory oversight that helps ensure medical products are safe and effective. In doing so, OTP strives to lead all regulatory decisions with data, impartiality, and compassion.
OTP oversees development for a wide variety of products including purified and recombinant proteins for hematology, antivenins, gene therapies, cell therapies, therapeutic tissue engineered products, human tissue products, therapeutic vaccines and other antigen-specific active immunotherapies, certain devices, and xenotransplantation products. Xenotransplantation is any procedure that involves the transplantation, implantation, or infusion of cells, tissues, or organs from a nonhuman animal source into a human recipient.
Interactions with OTP
Developers of advanced therapies regulated in OTP including cellular and gene therapy products have multiple opportunities to interact with OTP. These interactions may include both informal and formal meetings. Formal meetings follow established processes and timelines for requesting, scheduling, preparing, conducting, and documenting such meetings. These processes and timelines are defined in FDA regulations, guidance documents, and/or standard operating procedures. This website aims to provide resources for the types of potential meetings throughout the product lifecycle. Brief descriptions of the meeting types are provided below. Click on the links to access additional information.
This website provides information about possible meetings that the sponsor may have with OTP for biological products regulated under an IND/BLA pathway. For similar information for OTP regulated medical devices, please refer to Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program: Guidance for Industry and Food and Drug Administration Staff.
The format for a formal meeting may be face-to-face, teleconference, or Written Response Only (WRO). Face-to-face meeting includes in-person meetings and virtual meetings on IT platforms that allow for both audio and visual communication.
Informal interactions are interactions that are typically for simple clarifications and are not intended to be in lieu of formal meetings. OTP will consider such requests on a case-by-case basis. When deciding whether to engage in such informal interactions, OTP considers several factors, including the nature of the request and previous interactions with the office. The assigned Regulatory Project Manager is the point of contact for informal interaction requests.
The term “sponsor” refers to the entity that takes responsibility for and initiates a clinical investigation and a regulatory application, such as an IND. The sponsor may be an individual, pharmaceutical company, government agency, academic institution, or other organization. By extension, the “sponsor” is the entity that usually requests meetings with FDA about their product development.
Meetings Prior to Submission of an Investigational New Drug Application (IND)
When developing a novel product, a sponsor may want to obtain OTP’s advice on the data needed to support the submission of an Investigational New Drug application (IND). INTERACT and Pre-IND meetings may be held at these early stages of product development.
INTERACT Meeting
INTERACT or an INitial Targeted Engagement for Regulatory Advice on CBER/CDER ProducTs meeting is an opportunity for sponsors developing novel therapies to request feedback at an early stage of development. Before requesting an INTERACT meeting, a sponsor should have identified the investigational product to be evaluated in a clinical study and conducted some preliminary preclinical proof-of-concept studies with the intended investigational product, but not have designed and conducted definitive toxicology studies.
Find out more about OTP INTERACT Meetings
Note: If you are submitting a meeting request for a CDER-regulated product, contact druginfo@fda.hhs.gov for instructions on how to submit it to CDER.
Pre-IND Meeting
The primary purpose of a Pre-IND Meeting is to review and obtain feedback on the design of preclinical studies, the design of the initial IND study, and product manufacturing and quality controls needed to initiate human studies. The meeting may also provide an opportunity to discuss plans for studying the product in pediatric populations, the target product profile, the design and results of any natural history studies, and the best approach for presentation and formatting of data in the IND.
Find out more about Pre-IND Meetings
Meetings After Submission of an Investigational New Drug Application (IND)
Meetings between OTP and a sponsor at critical junctures during the IND phase provide an opportunity for OTP to provide valuable scientific and regulatory advice, with the goal of facilitating more efficient and robust product development programs. Types of meetings include End of Phase Meetings, Pre-BLA Meetings, and other meetings to discuss topics such as clinical protocol development, assay development, and product comparability studies.
FDA has established different categories of meetings, called Type A, B, C, and D Meetings. Each meeting type has specific timelines. These meeting types are explained in FDA guidance, Formal Meetings Between the FDA & Sponsors or Applicants of PDUFA Products Guidance for Industry. Furthermore, certain meetings held over the course of product development have been assigned to be either Type A, B, C, or D.
Type A Meetings
Type A Meetings are reserved for otherwise stalled product development programs to proceed or to address an important safety issue.
Find out more about Type A Meetings
Type B Meetings
IND End of Phase Meetings
The purpose of an End of Phase (EOP) Meeting is to review the overall status of the development program, including (but not limited to) the available clinical safety and efficacy data, and to address important questions or issues for all disciplines [e.g., Chemistry, Manufacturing, and Controls (CMC), pharmacology/toxicology (P/T), and clinical] that pertain to the further development of the product.
Find out more about IND End of Phase Meetings
Pre-BLA Meetings
The primary purpose of a Pre-BLA Meeting is to discuss the proposed content of the Biologics License Application.
Find out more about Pre-BLA Meetings
Breakthrough Therapy (BT) and Regenerative Medicine Advanced Therapy (RMAT)-Designated Products Meetings
Breakthrough Therapy (BT) Designation and Regenerative Medicine Advanced Therapy (RMAT) Designation are two of the expedited programs that may be applicable to advanced therapies such as cellular and gene therapy products that are intended to treat, modify, reverse, or cure a serious condition. For drugs developed under BT and RMAT, sponsors receive more intensive guidance on an efficient drug development program with increased interactions and communications with OTP.
Find out more about Meetings for Regenerative Medicine Advanced Therapy (RMAT) and Breakthrough Therapy (BT) Designated Products
Type C Meetings
A Type C Meeting is any meeting other than a Type A, Type B, Type B (EOP), or Type D Meeting regarding the development and review of a product.
Find out more about Type C Meetings
Type C Meetings to Discuss New Surrogate Endpoint(s)
This Type C Meeting is to facilitate early consultations on the use of a biomarker as a surrogate endpoint that has not been used previously as the primary basis for product approval in the proposed context of use. The purpose of this meeting is to discuss the feasibility of the surrogate as a primary endpoint and identify any gaps in knowledge and discuss how they might be addressed.
Find out more about Type C Meetings to Discuss New Surrogate Endpoint(s)
Type D Meetings
A Type D Meeting is focused on a narrow set of issues that are used to discuss issues at key decision points to provide timely feedback critical to move the program forward. The Type D Meeting should be limited to no more than two focused topics and should not require input from more than three disciplines. Requests could include the following:
- A follow-up question that raises a new issue after a formal meeting. (i.e., more than just a clarifying question about an FDA response from a prior meeting).
- A narrow issue on which the sponsor is seeking agency input with only a few (e.g., three to five questions total) associated questions.
- A general question about an innovative development approach that does not require extensive, detailed advice.
Find out more about Type D Meetings
Request for Clarifications to Formal Meetings
A Request for Clarification may be sought after a Type A, B, C, D and INTERACT meeting, to ensure the requestor’s understanding of FDA feedback provided in a preliminary response (if the formal meeting was canceled), meeting summary, or written response issued by FDA. Only questions of a clarifying nature should be submitted (i.e., to confirm something in meeting summary or in a WRO issued by the FDA) rather than new issues or new proposals.
Find out more about Requests for Clarification
Meetings After Approval of Biologics License Application
A meeting may be appropriate to obtain OTP input on planned BLA supplements, such as efficacy supplements or significant manufacturing changes.
Find out more about Meetings for Approved Products
Additional Information
Participation of Patients and Patient Advocates in Meetings
Patients and their advocates possess the unique, first-hand perspective of what it is like to live with or care for an individual with a disease, and the impact of available treatments on daily life. Patients can provide valuable input into the discussion of investigational therapies by describing their experience with a disease or condition, and by defining meaningful change in terms of their specific disease and the risks they are willing to accept. OTP welcomes the participation of patients and their advocates in formal meetings related to the development of investigational products. Sponsors who wish to include patients or their advocates in their meetings with OTP can directly invite and coordinate participation for such guests.
CBER Advanced Technologies Team (CATT) Meeting
A meeting with the CBER Advanced Technologies Team may be granted to prospective innovators/developers of advanced manufacturing and testing technologies to discuss a novel technology rather than a specific therapeutic product. CATT meetings focus on novel technologies that can have a significant impact on product development, manufacturing process and control strategies, and may also have regulatory implications. This includes manufacturing and analytical methods for those products or classes of products for which the Center has limited experience with the manufacturing or development process.
More information is available here: Link: CBER Advanced Technologies Team (CATT) | FDA
Meetings for Products Developed Outside of the United States
Requests for meetings about products that have been developed outside of the United States will be considered on a case-by-case basis to determine the most appropriate type of meeting.
If the intent of the meeting is to discuss submission of an IND for additional clinical studies in the US, then a Pre-IND Meeting is probably the most appropriate meeting type.
If the sponsor does not intend to submit an IND, but wants to discuss a future BLA submission, a Type C Meeting or a Pre-BLA Meeting may be appropriate, depending on the status of product development. We recommend that the sponsor/US Agent contact OTP at OTPRPMS@fda.hhs.gov for advice prior to submitting the meeting request.
References and Definitions
To help ensure an efficient meeting, OTP recommends that sponsors review the below applicable guidances, CBER SOPP and other resources.
- Glossary/Definitions
- Guidance: Formal Meetings Between the FDA & Sponsors or Applicants of PDUFA Products Guidance for Industry discusses the principles of good meeting management practices and describes standardized procedures for requesting, preparing, scheduling, conducting, and documenting such formal meetings.
- SOPP 8101.1 Regulatory Meetings with Sponsors and Applicants for Drugs & Biological Products serves as a guide for CBER staff for scheduling and conducting regulatory meetings between individuals in CBER and representatives of the regulated industry (including sponsors/applicants of user fee related products) and /or individual sponsor-investigators to address issues to product development.
- Guidance: Best Practices for Communication Between IND Sponsors and FDA During Drug Development describes best practices and procedures for timely, transparent, and effective communications between IND sponsors and FDA at critical junctures in drug development, which may facilitate earlier availability of safe and effective drugs to the American public.
- Guidance: Rare Diseases: Early Drug Development and the Role of Pre-IND Meetings describes frequently encountered issues to consider in early drug development and pre-IND meetings, including topics related to pharmaceutical quality, nonclinical evaluation, clinical pharmacology, and clinical development including early phase study designs and statistical analysis plans.
- OTP Learn webinar: OTP Learn