Medical Products for Newborns
The first several weeks of life is a time of critical growth and development for babies. Newborns undergo rapid changes in all their organ systems and tissues, making them a uniquely vulnerable population. Medical products approved by the FDA for newborn babies must be proven to be safe and effective.
Medical products need to be studied in neonatal clinical trials. The growth and developmental changes that occur during the newborn period (the first 28 days of life for full-term babies) can affect the safety, effectiveness and dosing of medicines when used in newborns. Medical devices for newborns need to be designed taking into consideration a newborn’s size, growth, activity and physiologic changes. Newborns’ responses to medical products often can’t be predicted from data collected in studies of adults.
Long-term safety studies are often important to assess the safety of medical products used in newborns because important health effects resulting from medical products used during the newborn period may not become apparent until after a baby has grown older.
Federal laws incentivize and require pediatric studies under certain circumstances but there are still many challenges for addressing the needs of newborns. For example, most drugs used in neonatal intensive care units have not been approved by the FDA for use in newborns. Doctors routinely treat newborns with medicines approved for adults or older children if the doctor believes the medicine is the best available treatment option for the newborn. Additionally, many medical conditions that occur in newborns don’t occur in older children or adults, so data from pediatric or adult studies often are not available to help inform the assessment of safety and effectiveness of medical products for these conditions.
Labeling changes for medical products for children and newborns
Advancing treatments for newborns and including evidence-based information in product labeling about how to safely treat newborns continues to be a key area of focus for the FDA. For example, the agency:
- Supports medical product development by providing neonatal-perinatal medicine expertise across the agency
- Explores neonatology considerations raised by pediatric studies required or requested under federal laws including the Pediatric Research Equity Act and the Best Pharmaceuticals for Children Act
- Trains staff across the agency and provides expertise to external stakeholders on issues in neonatal medical product development
- Advances regulatory science for clinical trials involving neonates to support the agency’s decision-making
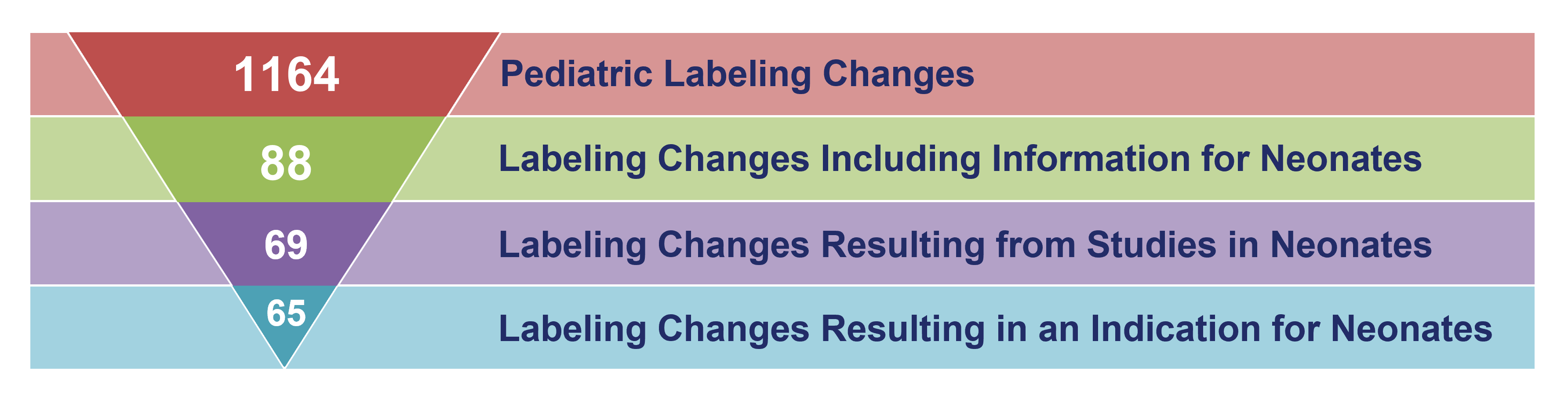
Labeling Changes for Neonates 1999 – 2023
The table below includes labeling changes for pediatric and neonatal drugs under certain federal laws. The number of pediatric labeling changes includes each individual label change approved by the FDA. One product may undergo several pediatric labeling changes. For example, a product’s labeling may be updated once when studies in older children have been reviewed by the FDA and again years later after studies in younger children are completed. Many labeling changes represent a new FDA approval for children. Others provide new safety or efficacy information about how to treat children. Drugs may be labeled with information for neonates even if studies were not conducted in neonates. For example, drugs may be labeled with safety information based on non-clinical data.
International Neonatal Consortium
The FDA participates in the International Neonatal Consortium (INC), a global collaboration formed to forge a predictable regulatory path for evaluating the safety and effectiveness of medical products for neonates. The consortium has spearheaded many opportunities to improve and accelerate neonatal product development. For example, the INC developed a neonatal adverse event severity scale to help improve the quality of safety evaluations in neonatal clinical trials.
Additional resources
- Final guidance: Considerations for Long-Term Clinical Neurodevelopmental Safety Studies in Neonatal Product Development
- Guidance snapshot and podcast (podcast transcript)
- Final guidance: General Clinical Pharmacology Considerations for Neonatal Studies for Drugs and Biological Products
- Draft guidance: General Clinical Pharmacology Considerations for Pediatric Studies of Drugs, Including Biological Products
- Draft guidance: Measuring Growth and Evaluating Pubertal Development in Pediatric Clinical Trials