Pediatric Labeling Changes
Having information about a medicine’s safety, effectiveness and dosing for children in the product’s labeling is important to ensure health care professionals can make evidence-based decisions about treating children. A pediatric labeling change refers to any update to a product’s labeling to add information about safety, effectiveness or dosing for children. Many labeling changes represent a new FDA approval for children. Others provide new safety or efficacy information about how to treat children. For example, some labeling changes may reflect dosing information for children or new safety information advising against use in certain pediatric populations.
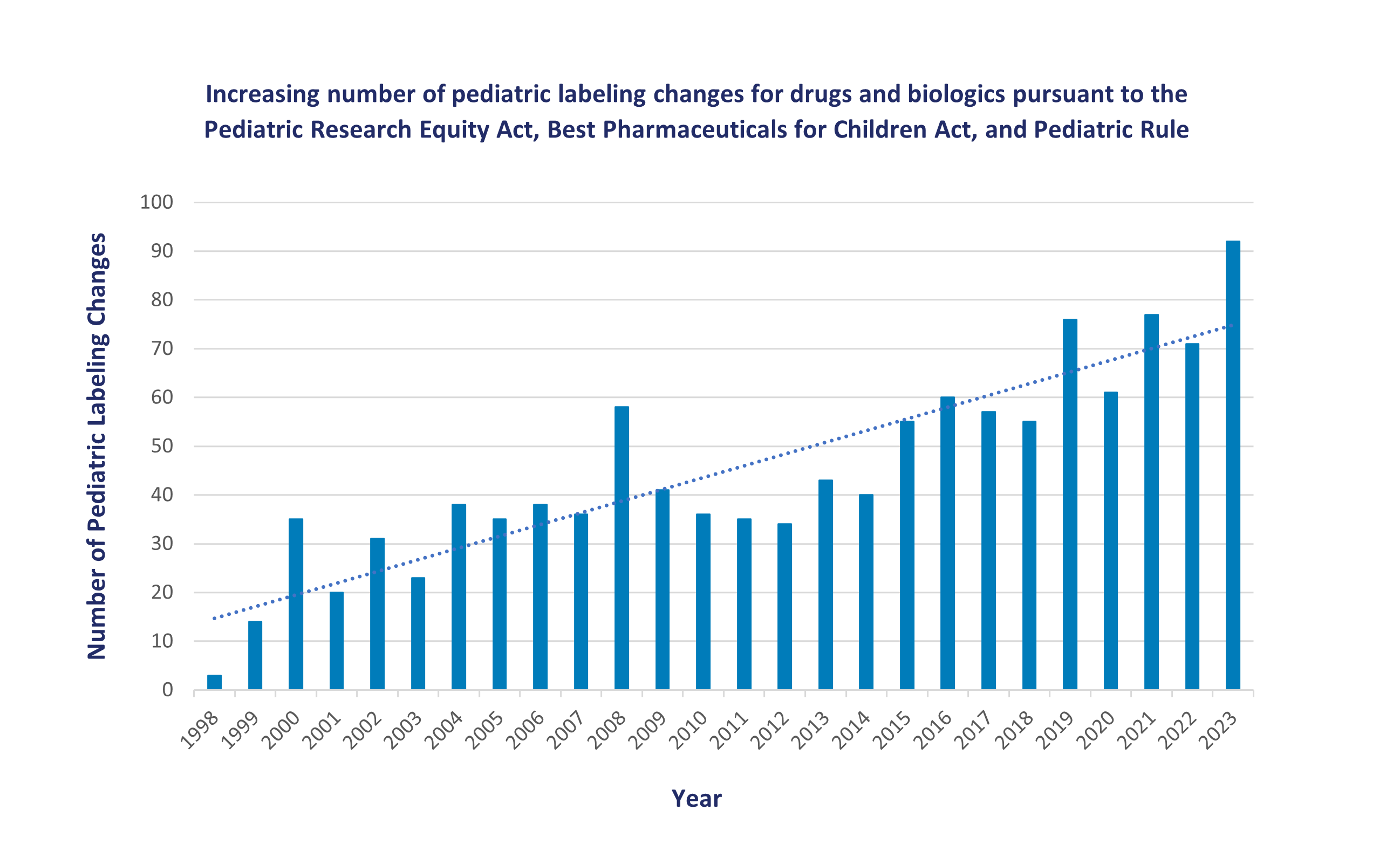
The pediatric labeling changes and study characteristics data presented below highlight key pediatric information obtained from studies submitted to the FDA in response to legislative initiatives, including the Pediatric Research Equity Act (PREA) of 2003, the Best Pharmaceuticals for Children Act (BPCA) of 2002 and the Pediatric Rule of 1998. These laws encourage and, in certain circumstances, require pharmaceutical companies to develop medicines for children. These laws have led to a dramatic increase in pediatric studies submitted to the FDA and resulted in new treatment options for children.
Pediatric Labeling Changes 1998 – 2023
The graph below shows the number of labeling changes for pediatric populations under certain federal laws.
The number of pediatric labeling changes includes each individual label change approved by the FDA. One product may undergo several pediatric labeling changes. For example, a product’s labeling may be updated once when studies in older children have been reviewed by the FDA and again years later after studies in younger children are completed.
Pediatric Labeling Changes Spreadsheet
This spreadsheet contains all pediatric labeling changes made under BPCA, PREA and the Pediatric Rule since 1998. Each pediatric labeling change includes the date of the pediatric labeling change, specific drug or biological product, indication(s) studied, a summary of the labeling change, therapeutic category and type of legislation.
Pediatric Labeling Changes Spreadsheet (XLSX - 311 KB)
Pediatric Study Characteristics Spreadsheet
This spreadsheet contains pediatric study characteristics for the clinical trials conducted to support each pediatric labeling change, including the study number, type of study, study design, number of pediatric participants, ages studied, number of study centers, number of countries and, for BPCA clinical trials, any available racial and ethnic information. The spreadsheet includes pediatric studies that resulted in labeling changes made since September 27, 2007 as required by the FDA Amendments Act of 2007.
Pediatric Descriptors Spreadsheet (XSLX- 425 KB)
FDA intends to update these spreadsheets quarterly.