3D Printing in FDA’s Rapid Response to COVID-19
Updates on activities performed under the FDA, NIH, and VA Memorandum of Understanding in collaboration with America Makes.
Assessing the role of additive manufacturing in support of the U.S. COVID-19 response
The FDA continues to take creative and flexible approaches to address availability of critical medical products in response to the COVID-19 pandemic. In addition, non-traditional manufacturers and community responders have helped address shortages and gaps in medical supplies during the COVID-19 pandemic, and yielded millions of pieces of equipment and supplies, such as masks, face shields, and other 3D-printed medical devices. In September 2020, the FDA funded a study, conducted by America Makes, to summarize the impact of 3D printing on the overall COVID-19 response. The FDA has now made publicly available this report on the use of additive manufacturing by non-traditional producers in support of the U.S. COVID-19 response. The study shows successes, challenges, and key lessons learned to build on and improve future crisis response. The FDA is reviewing the report to assess gaps in the response and potential mitigations for future public health emergencies.
America Makes report links (posted July 9, 2021):
- Executive Summary: Assessing the Role of Additive Manufacturing in Support of the U.S. COVID-19 Response (PDF, 1.2 MB)
- Assessing the Role of Additive Manufacturing in Support of the U.S. COVID-19 Response - An impact survey of interagency 3D Printing response efforts (PDF, 3 MB) - How did 3D printing help consumers, responders, and healthcare workers during the COVID-19 pandemic? This report examines actions taken between February 15, 2020 – July 15, 2020.
Update - November 2021: Trust in the Time of Covid-19: 3D Printing and Additive Manufacturing (3DP/AM) as a Solution to Supply Chain Gaps - FDA and partners published a commentary in NEJM Catalyst Innovations in Care Delivery on how a public-private collaboration, Covid 3D TRUST, has helped to address critical supply shortages by empowering designers, manufacturers, and users of 3D-printed PPE during the COVID-19 pandemic.
The FDA continues to take creative and flexible approaches to address access to critical medical products in response to COVID-19. Researchers at academic institutions, non-traditional manufacturers, communities of makers, and individuals are banding together to support and fill local and national needs. The FDA is actively engaged across this spectrum and developing ways to assist and support people who are looking to help their communities in these ways. Our goal is to help expand the availability of certain products in ways that are consistent with FDA’s public-health mission.
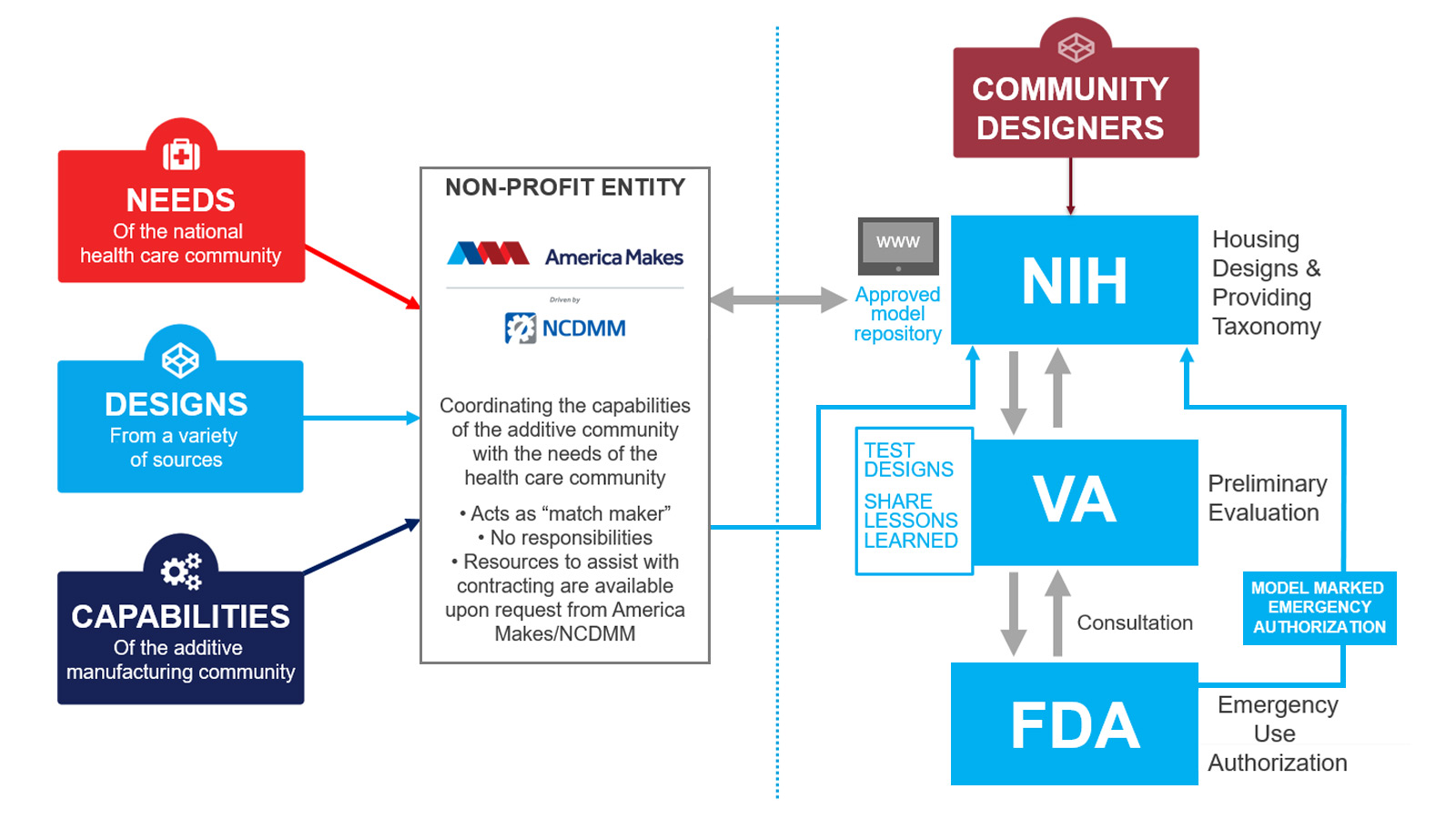
For example, the FDA is working in partnership with the NIH, VA, and America Makes to support non-traditional manufacturing approaches, such as 3D printing, to address devices shortages including personal protective equipment (PPE). Through this partnership, 3D-printable designs for COVID response are given a clinical assessment by the VA and the NIH posts them on the 3D Print Exchange. FDA has, among other things, provided information on labeling and testing for face shields and face masks.
This page provides an update on how this partnership has contributed to the number of medical devices—including PPE—and parts available to support the COVID-19 response since it was launched in March 2020. For example, 31 community-submitted designs passed the testing performed by VA clinics and were given clinically reviewed status in the first three months. In addition, this effort has so far matched more than 500,000 3D-printed face shields and more than 348,000 3D-printed face masks with health care providers and others in need. FDA has issued a temporary policy for face masks and respirators during the COVID-19 public-health emergency.
The workflow is structured as follows:
- Healthcare organizations and hospitals, designers, and manufacturing facilities can all submit their information to America Makes to connect local health care organizations in need with nearby design and manufacturing capabilities. New designs may be sent to the NIH 3D Print Exchange (NIH 3DPX) for clinical review if appropriate.
- Alternatively, designers and community members can also submit 3D-printable files directly to the NIH 3DPX.
- Once posted, the VA will perform preliminary evaluations and experimental tests on selected designs to assess their appropriateness for use in a clinical setting. Those that pass receive an NIH-issued clinical badge.
- The smaller number of designs given a clinically reviewed badge on the NIH 3DPX, after testing by VA, helps healthcare groups, stakeholders, and community members choose high-quality designs to 3D print for PPE and other products to support the COVID-19 response.
- Some designs are for products that may also receive Emergency Use Authorization by the FDA, and that is noted by a separate badge on the NIH 3DPX. (Manufacturers are responsible for understanding FDA’s requirements, including obtaining an EUA as appropriate, and may want to review the policies described in FDA’s COVID-19 guidances.)
Since the official start of this collaboration on March 25, 2020, each partner has made huge efforts to continuously meet their goals, and collectively make significant contributions to the number of medical devices—including PPE—and parts available to support the COVID-19 response.
Recent events
- The Fit-to-Face Mask Design Challenge organized by America Makes in collaboration with the Department of Veterans Affairs called on all innovators and designers to quickly create and/or modify 3D-printed face mask designs to fit a wide range of faces while improving continuous fit-to-face contact and providing appropriate sealing between the skin and mask. The Top Designs were announced on May 12, 2020.
- VHA Innovation Ecosystem and Challenge America launched their 2nd COVID-19 Maker Challenge series of five COVID-19 Maker Challenges. These are focused on finding solutions for potential COVID-related challenges for environmental management services. The goal of the challenge is to enlist the talents of engineers, designers, and other professionals to create innovative solutions to the challenges confronting these essential workers in their efforts to fight COVID-19. America Makes is a sponsor of the COVID-19 Maker Challenge Series.
- On May 15, 2020, the FDA held a Virtual Public Town Hall on 3D printed swabs which provided information and answered questions about swabs used for diagnostic purposes during the COVID-19 pandemic.
Activities from each agency and collaborator
The Food and Drug Administration entered into this interagency MOU to support the use of advanced manufacturing technologies such as 3D printing to respond to the COVID-19 pandemic. FDA is actively working with the members of the MoU collaboration and non-profit partner America Makes on communications and criteria for evaluating open source designs that have been uploaded to the NIH 3DPX. The collaboration does not include any confidential information or regulatory submissions, which are being handled through standard processes. In addition, the FDA is communicating with stakeholder organizations regarding access to medical products in response to the COVID-19 pandemic. As of June 9, 2020, FDA has:
- Fielded hundreds of inquiries about 3D-printable PPE, swabs, and other medical devices
- Developed frequently asked questions and other documents
- Consulted daily with NIH, VA, and America Makes on best practices, considerations, and other key information for 3D printing medical products
- Held and attended more than 50 stakeholder meetings and seminars on use of 3D printing
The National Institutes of Health 3D Print Exchange (NIH 3DPX) has been the primary conduit for digital models that can be posted, used, and clinically reviewed by VA for appropriateness. Since March 27, 2020, the NIH 3D Print Exchange website has been consistently used as a community resource. (Numbers in this section updated as of June 9, 2020, unless otherwise noted.)
- Models Published: 685 total (as of August 3, 2020)
- Clinically Reviewed: 33 (as of August 3, 2020)
- Community Use: 28
- Prototypes: 484
- Warning: 33
- Visitors:
- 493,000 unique visitors to the 3D Print Exchange
- COVID-19 main page has over 224,000 views since launch
- Model page views: (including increases from previous week)
- Total collective views all models: 1,112,651 (+23,114)
- Comments all: 487
- Confirmed Builds: 145 (+10)
- Downloads:
- 95,743 files (a 2,500% increase since the equivalent time period prior to launching the COVID collection)
- Surgical mask tension release: 19,819
- Stopgap surgical face mask: 18,952
- DtM-v3.1 Face Shield: 9,782
The Veterans Health Administration Innovation Ecosystem has been designing models to directly address COVID-19 PPE demand from health care providers and other needs during the response. They have also been tapping their network of engineers and clinicians to field test selected devices submitted to the site for clinical use to support the “clinically reviewed” badge that those devices receive from the VA. VA personnel perform preliminary evaluations and then experimental tests according to existing standards on selected designs to assess their appropriateness for use in a clinical setting. Those that pass receive a clinical badge. Lastly, they are also developing additional testing and clinical evaluation protocols for clinical use designs.
- PPE designs include the Stopgap surgical face mask and Surgical mask tension release
- VA personnel have reviewed hundreds of prototype designs to arrive at the 18 clinically reviewed designs on the 3D Print Exchange (as of June 9, 2020)
America Makes is connecting health care providers with manufacturers that have the capacity to manufacture medical products for use during the COVID-19 response. They are also meeting regularly with interagency partners and stakeholders to identify and fill gaps in the supply chain. The America Makes website is a principal connection hub and source of information. Website and group activities include:
- Manufacturers submitting capabilities information: 480 (as of June 9, 2020)
- Face shields/comfort straps only: 222
- Face shields and/or face masks: 47
- Other: 211
- Total pieces matched: 943,678+ (as of August 3, 2020)
- Face shields: 540,470
- Face masks (non-N95): 348,361
Additional FDA Activities
The FDA continues to issue Guidance Documents, web publications, Emergency Use Authorizations, and other communications concerning COVID-19. A full list of FDA COVID-19 activities can be found on the Agency’s COVID-19 website.