Drug Trials Snapshots: PLUVICTO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable). Refer to the PLUVICTO Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
PLUVICTO (lutetium Lu 177 vipivotide tetraxetan)
(ploo vik' toe)
Advanced Accelerator Applications USA
Approval date: March 23, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PLUVICTO is used to treat adults with a certain type of advanced prostate cancer called prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC) that is metastatic (this means that it has spread to other parts of the body) and that has already been treated with other anti-cancer treatments.
How is this drug used?
PLUVICTO is given by a healthcare provider directly into a vein (intravenous) every six weeks for up to six doses.
Who participated in the clinical trials?
The FDA approved PLUVICTO based on evidence from one clinical trial (NCT03511664) of 831 patients with PSMA-positive mCRPC. The safety population of this trial included 734 patients. The trial was conducted in Canada, Europe, and the United States.
How were the trials designed?
The benefits and side effects of PLUVICTO were evaluated in one clinical trial of 831 patients with PSMA-positive mCRPC who had already been treated with other anti-cancer treatments.
Patients were randomly assigned to receive PLUVICTO plus best standard of care (BSoC) or BSoC alone. Patients received PLUVICTO 7.4 GBq (200 mCi) intravenously every six weeks for up to six doses. The treatment continued until the disease progressed or unacceptable side effects.
The benefit of PLUVICTO was assessed by measuring the length of time that the patient was still alive (overall survival).
How were the trials designed?
The efficacy and safety of PLUVICTO were evaluated in one randomized, multicenter, open-label trial in male patients with progressive, PSMA-positive mCRPC who had been treated with androgen receptor pathway inhibition and taxane-based chemotherapy. Patients were randomized 2:1 to receive PLUVICTO 7.4 GBq plus BSoC or BSoC alone. PLUVICTO was given intravenously every 6 weeks for up to 6 doses. Treatment continued until radiographic disease progression, unacceptable toxicity, or withdrawal.
The primary efficacy endpoint was overall survival, defined as the time from randomization to the time of death due to any cause.
DEMOGRAPHICS SNAPSHOT
Figure 1. Baseline Demographics by Sex (Intent-to-Treat Population*)
Source: Adapted from FDA Review
* The safety population of this trial included 734 male patients; 97 patients were not treated.
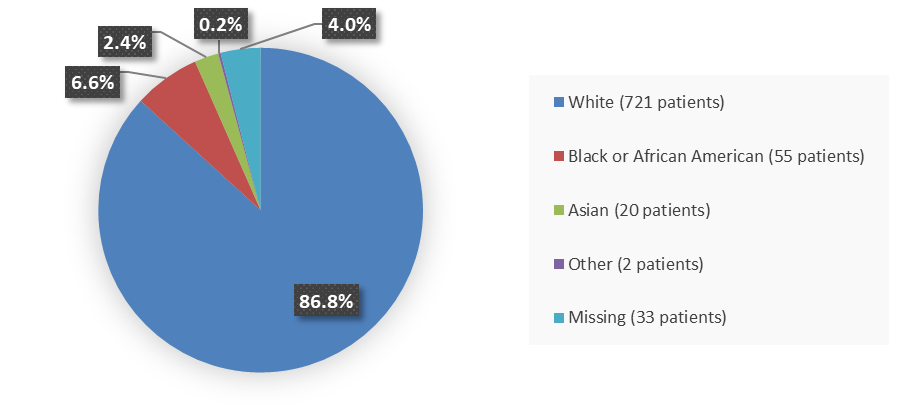
Figure 2. Baseline Demographics by Race (Intent-to-Treat Population)
Source: Adapted from FDA Review
Other includes Native Hawaiian or Other Pacific Islander, American Indian or Alaska Native, and more than one race reported.
Figure 4. Baseline Demographics by Age (Intent-to-Treat Population)
Source: Adapted from FDA Review
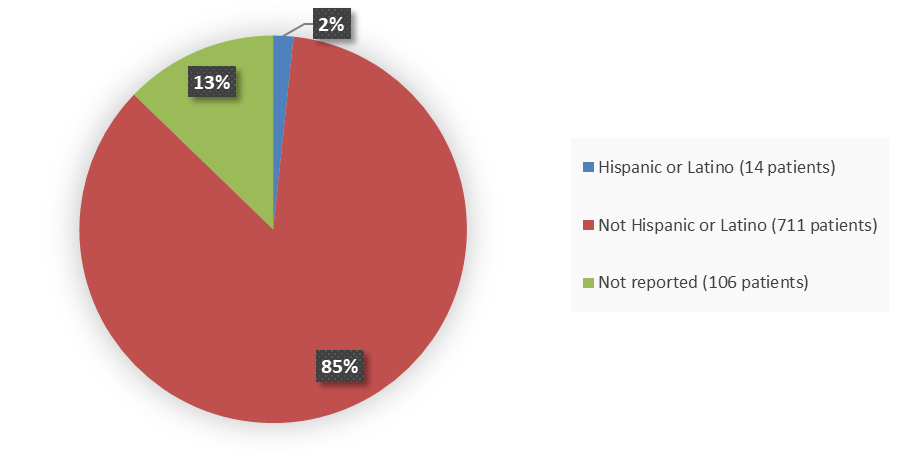
Figure 6. Baseline Demographics by Ethnicity (Intent-to-Treat Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Demographics of the Intent-to-Treat Population
| Demographic | PLUVICTO Plus BsoC N=551 n (%) |
BsoC N=280 n (%) |
Total N=831 n (%) |
|---|---|---|---|
| Sex | |||
| Male | 551 (100) | 280 (100) | 831 (100) |
| Race1 | |||
| White | 486 (88.2) | 235 (83.9) | 721 (86.8) |
| Black or African American | 34 (6.2) | 21 (7.5) | 55 (6.6) |
| Asian | 9 (1.6) | 11 (3.9) | 20 (2.4) |
| Other | 2 (0.4) | 0 | 2 (0.2) |
| Missing | 20 (3.6) | 13 (4.6) | 33 (4.0) |
| Age group, years | |||
| <65 | 145 (26.3) | 60 (21.4) | 205 (24.7) |
| ≥65 | 406 (73.7) | 220 (78.6) | 626 (75.3) |
| Age, years | |||
| Mean | 69.7 | 70.5 | 70.0 |
| SD | 7.41 | 7.80 | 7.55 |
| Median | 70.0 | 71.5 | 71.0 |
| Min, max | 48, 94 | 40, 89 | 40, 94 |
| Ethnicity | |||
| Hispanic or Latino | 11 (2.0) | 3 (1.1) | 14 (1.7) |
| Not Hispanic or Latino | 471 (85.5) | 240 (85.7) | 711 (85.6) |
| Not reported | 69 (12.5) | 37 (13.2) | 106 (12.8) |
| Region | |||
| North America | 393 (71.3) | 209 (74.6) | 602 (72.4) |
| Europe | 158 (28.7) | 71 (25.4) | 229 (27.6) |
Source: Adapted from FDA Review
1 Other includes Native Hawaiian or Other Pacific Islander, American Indian or Alaska Native and more than one race reported.
Abbreviations: BSoC, best standard of care; SD, standard deviation
Table 2. Demographics of the Safety Population
| Demographic | PLUVICTO Plus BSoC N=529 n (%) |
BSoC N=205 n (%) |
Total N=734 n (%) |
|---|---|---|---|
| Sex | |||
| Male | 529 (100) | 205 (100) | 734 (100) |
| Race1 | |||
| White | 465 (87.9) | 173 (84.4) | 638 (86.9) |
| Black or African American | 34 (6.4) | 19 (9.3) | 53 (7.2) |
| Asian | 9 (1.7) | 8 (3.9) | 17 (2.3) |
| Other | 2 (0.4) | 0 | 2 (0.3) |
| Missing | 19 (3.6) | 5 (2.4) | 24 (3.3) |
| Age group, years | |||
| <65 | 142 (26.8) | 42 (20.5) | 184 (25.1) |
| ≥65 | 387 (73.2) | 163 (79.5) | 550 (74.9) |
| Age, years | |||
| Mean | 69.6 | 70.5 | 69.8 |
| SD | 7.41 | 7.76 | 7.51 |
| Median | 70.0 | 71.0 | 70.0 |
| Min, max | 48, 94 | 40, 89 | 40, 94 |
| Ethnicity | |||
| Hispanic or Latino | 11 (2.1) | 2 (1.0) | 13 (1.8) |
| Not Hispanic or Latino | 457 (86.4) | 171 (83.4) | 628 (85.6) |
| Not reported | 61 (11.5) | 32 (15.6) | 93 (12.7) |
| Region | |||
| North America | 381 (72.0) | 144 (70.2) | 525 (71.5) |
| Europe | 148 (28.0) | 61 (29.8) | 209 (28.5) |
Source: Adapted from FDA Review
1 Other includes Native Hawaiian or Other Pacific Islander, American Indian or Alaska Native and more than one race reported.
Abbreviations: BSoC, best standard of care; SD, standard deviation
What are the benefits of this drug?
In patients with positive expression of PSMA after previous treatment with other anti-cancer treatments, PLUVICTO increased patients’ survival (overall survival). Men who received PLUVICTO plus BSoC lived about 15 months compared to 11 months for patients taking BSoC alone.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary endpoint was overall survival defined as the time from randomization to the time of death due to any cause.
Table 3 shows overall survival efficacy results for the patients evaluated in the clinical trial.
Table 3. Overall Survival Efficacy Results in Patients With PSMA-Positive mCRPC (Intent-to-Treat Population)
| Overall Survival | PLUVICTO Plus BSoC N=551 |
BSoC N=280 |
|---|---|---|
| Number of deaths, n (%) | 343 (62) | 187 (67) |
| Median, months (95% CI)1 | 15.3 (14.2, 16.9) | 11.3 (9.8, 13.5) |
| Hazard ratio (95% CI)2 | 0.62 (0.52, 0.74) | |
| P-value3 | <0.001 | |
Source: PLUVICTO Prescribing Information
1 Based on Kaplan-Meier estimate.
2 Hazard ratio is based on a Cox regression model (with treatment as the only covariate) stratified by baseline lactate dehydrogenase (LDH) (≤260 IU/L vs. >260 IU/L), presence of liver metastases (yes vs. no), Eastern Cooperative Oncology Group performance status (ECOG PS) score (0-1 vs. 2) and inclusion of an androgen receptor pathway inhibitor (ARPI) as part of BSoC at the time of randomization (yes vs. no).
3 P-value is based on a stratified log-rank test by baseline LDH (≤260 IU/L vs. >260 IU/L), presence of liver metastases (yes vs. no), ECOG PS score (0-1 vs. 2) and inclusion of an ARPI as part of BSoC at the time of randomization (yes vs. no).
Abbreviations: BSoC, best standard of care; CI, confidence interval; mCRPC, metastatic castration-resistant prostate cancer; PSMA, prostate-specific membrane antigen
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All the patients were male since PLUVICTO is for the treatment of prostate cancer.
- Race: PLUVICTO worked similarly in White patients and Black or African American patients. The number of patients in other races was limited; therefore, differences in how PLUVICTO worked among other races could not be determined.
- Age: PLUVICTO worked similarly in patients younger than 65 years of age and in patients 65 years of age and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Overall survival efficacy results by demographic subgroups are presented in Table 4.
Table 4. Overall Survival Efficacy Results in Demographic Subgroups (Intent-to-Treat Population)
| Demographic | PLUVICTO Plus BSoC n/N (%) |
BSoC n/N (%) |
HR | 95% CI |
|---|---|---|---|---|
| Race | ||||
| White | 300/486 (61.7) | 159/235 (67.7) | 0.63 | 0.52, 0.77 |
| Black or African American | 20/34 (58.8) | 12/21 (57.1) | 0.60 | 0.29, 1.24 |
| Asian | 9/9 (100) | 7/11 (63.6) | 1.04 | 0.38, 2.81 |
| Age, years | ||||
| <65 | 82/145 (56.6) | 38/60 (63.3) | 0.73 | 0.49, 1.10 |
| ≥65 | 261/406 (64.3) | 149/220 (67.7) | 0.59 | 0.48, 0.73 |
Source: Adapted from FDA Review
Abbreviations: BSoC, best standard of care; CI, confidence interval; HR, hazard ratio; n/N, number of events/number of patients
What are the possible side effects?
PLUVICTO is a radioactive drug which may increase the risk of lifetime radiation exposure.
PLUVICTO may cause serious side effects including reductions in blood cell counts (red cells, white cells, and platelets) and reduced kidney function.
The most common side effects of PLUVICTO were fatigue, dry mouth, nausea, anemia, decreased appetite, and constipation.
What are the possible side effects (results of trials used to assess safety)?
Table 5 summarizes adverse reactions in patients with PSMA-positive mCRPC (safety population).
Table 5. Adverse Reactions (≥5%) in Patients With PSMA-Positive mCRPC Who Received PLUVICTO Plus BSoC (Safety Population)
| Adverse Reactions | PLUVICTO Plus BSoC N=529 |
BSoC N=205 |
||
|---|---|---|---|---|
| All Grades % |
Grades 3 to 4 % |
All Grades % |
Grades 3 to 4 % |
|
| General disorders | ||||
| Fatigue | 43 | 6 | 23 | 1.5 |
| Decreased appetite | 21 | 1.9 | 15 | 0.5 |
| Weight decreased | 11 | 0.4 | 9 | 0 |
| Peripheral edema1 | 10 | 0.4 | 7 | 0.5 |
| Pyrexia | 7 | 0.4 | 3.4 | 0 |
| Gastrointestinal disorders | ||||
| Dry mouth2 | 39 | 0 | 0.5 | 0 |
| Nausea | 35 | 1.3 | 17 | 0.5 |
| Constipation | 20 | 1.1 | 11 | 0.5 |
| Vomiting3 | 19 | 0.9 | 6 | 0.5 |
| Diarrhea | 19 | 0.8 | 2.9 | 0.5 |
| Abdominal pain4 | 11 | 1.1 | 6 | 0.5 |
| Blood and lymphatic system disorders | ||||
| Anemia | 32 | 13 | 13 | 4.9 |
| Thrombocytopenia | 17 | 8 | 4.4 | 1 |
| Renal and urinary disorders | ||||
| Urinary tract infection5 | 12 | 3.8 | 1 | 0.5 |
| Acute kidney injury6 | 9 | 3.2 | 6 | 2.9 |
| Nervous system disorders | ||||
| Dizziness | 8 | 0.9 | 4.4 | 0 |
| Headache | 7 | 0.8 | 2 | 0 |
| Dysgeusia7 | 7 | 0 | 1.5 | 0 |
Source: PLUVICTO Prescribing Information
Grading according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0
1 Peripheral edema includes peripheral edema, fluid retention, and fluid overload.
2 Dry mouth includes dry mouth, aptyalism, and dry throat.
3 Vomiting includes vomiting and retching.
4 Abdominal pain includes abdominal pain, abdominal pain upper, abdominal discomfort, abdominal pain lower, abdominal tenderness, and gastrointestinal pain.
5 Urinary tract infection includes urinary tract infection, cystitis, and cystitis bacterial.
6 Acute kidney injury includes blood creatinine increased, acute kidney injury, renal failure, and blood urea increased.
7 Dysgeusia includes dysgeusia and taste disorder.
Abbreviations: BSoC, best standard of care; mCRPC, metastatic castration-resistant prostate cancer; PSMA, prostate-specific membrane antigen
Clinically relevant adverse reactions in less than 5% of patients who received PLUVICTO plus BSoC included dry eye, vertigo, and pancytopenia (including bicytopenia).
Table 6. Select Laboratory Abnormalities (≥10%) That Worsened From Baseline in Patients With PSMA-Positive mCRPC Who Received PLUVICTO Plus BSoC (Between Arm Difference of ≥5% All Grades) (Safety Population)
| Laboratory Abnormalities | PLUVICTO Plus BSoC1 | BSoC2 | ||
|---|---|---|---|---|
| All Grades % |
Grades 3 to 4 % |
All Grades % |
Grades 3 to 4 % |
|
| Chemistry | ||||
| Decreased calcium | 39 | 2.5 | 28 | 3 |
| Decreased sodium | 33 | 0.63 | 23 | 1 |
| Increased aspartate aminotransferase | 28 | 1.1 | 18 | 13 |
| Increased creatinine | 24 | 0.93 | 14 | 0.53 |
| Increased potassium | 24 | 0.6 | 18 | 0.53 |
| Increased sodium | 11 | 03 | 5 | 03 |
| Hematology | ||||
| Decreased lymphocytes | 85 | 47 | 51 | 18 |
| Decreased hemoglobin | 63 | 153 | 34 | 73 |
| Decreased leukocytes | 56 | 7 | 22 | 2 |
| Decreased platelets | 45 | 9 | 20 | 2.5 |
| Decreased neutrophils | 28 | 4.5 | 9 | 0.5 |
Source: PLUVICTO Prescribing Information
1 The denominator used to calculate the rate for each laboratory parameter varied from 506 to 529 based on the number of patients with a baseline value and at least one post-treatment value.
2 The denominator used to calculate the rate for each laboratory parameter varied from 194 to 198 based on the number of patients with a baseline value and at least one post-treatment value.
3 No Grade 4 laboratory abnormalities worsening from baseline were reported.
Abbreviations: BSoC, best standard of care; mCRPC, metastatic castration-resistant prostate cancer; PSMA, prostate-specific membrane antigen
Were there any differences in side effects among sex, race and age?
- Sex: All the patients were male since PLUVICTO is for the treatment of prostate cancer.
- Race: The occurrence of side effects was similar in White patients and Black or African American patients. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among other races could not be determined.
- Age: The occurrence of side effects was similar in patients younger than 65 years of age and in patients 65 years of age and older.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Analysis of treatment-emergent adverse events by demographic subgroups was limited to age and race due to the fact that all patients in the trial were male and the vast majority were White and 65 years of age and older.
Table 7. Treatment-Emergent Adverse Events by Race (Safety Population)
| Adverse Event | PLUVICTO Plus BSoC | BSoC | ||||
|---|---|---|---|---|---|---|
| White N=465 n (%) |
Black or African American N=34 n (%) |
Asian N=9 n (%) |
White N=173 n (%) |
Black or African American N=19 n (%) |
Asian N=8 n (%) |
|
| AEs | 456 (98.1) | 34 (100.0) | 8 (88.9) | 146 (84.4) | 14 (73.7) | 6 (75.0) |
| Grade 3 to 5 AEs | 247 (53.1) | 16 (47.1) | 3 (33.3) | 66 (38.2) | 7 (36.8) | 4 (50.0) |
| SAEs | 171 (36.8) | 10 (29.4) | 3 (33.3) | 50 (28.9) | 5 (26.3) | 2 (25.0) |
Table 8. Treatment-Emergent Adverse Events by Age (Safety Population)
| Adverse Event | PLUVICTO Plus BSoC | BSoC | ||
|---|---|---|---|---|
| Age <65 N=142 n (%) |
Age ≥65 N=387 n (%) |
Age <65 N=42 n (%) |
Age ≥65 N=163 n (%) |
|
| AEs | 136 (95.8) | 383 (99.0) | 30 (71.4) | 140 (85.9) |
| Grade 3 to 5 AEs | 72 (50.7) | 207 (53.5) | 13 (31.0) | 65 (39.9) |
| SAEs | 45 (31.7) | 147 (38.0) | 7 (16.7) | 50 (30.7) |
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.