Drug Safety Priorities 2016: Initiatives and Innovation
Center for Drug Evaluation and Research
U. S. Food and Drug Administration
Print Version (PDF - 2.3MB)
CONTENTS
- Introduction
- Drug Safety Oversight Across the Product Lifecycle
- Advancing Drug Safety Science
- Improving Operations and Management in Support of Drug Safety
- Communicating Drug Safety: A Global Public Interface
Introduction
For more than a century, the safety of medicines approved for use in the United States has been one of the mission cornerstones of the Food and Drug Administration (FDA). From the Food and Drug Act of 1906, through legislation enacted over the years, FDA’s regulatory authorities and resources have been progressively enhanced, allowing the Agency to consistently strengthen and modernize its drug safety initiatives and programs.
With millions of Americans taking more medicines than at any time in history, an unanticipated drug safety problem can rapidly escalate to become a major public health threat—demanding a multifaceted safety effort based on interdisciplinary scientific teamwork that enables rapid but balanced regulatory and public health decision making. Drug Safety Priorities: Initiatives and Innovation describes a number of programs that assure the safety of drug products, depicting the extensive, interrelated, and cooperative nature of our drug safety enterprise, and also reports on key safety milestones achieved over 2015 and to date in 2016.
This report goes beyond previously issued safety-related reports to offer a broader picture of FDA’s safety efforts, highlighting the critical advantages gained through a range of multidisciplinary collaborations and partnerships. From innovative computing platforms that support new drug reviews, to the ongoing implementation of existing programs like Safety First and the Safe Use Initiative, the active surveillance capacities of the Sentinel System, improvements in drug product quality oversight, and state-of-the-art communications tools that exploit new opportunities in social media and mobile device applications, FDA is evolving a drug safety enterprise that leverages gains achieved within the Agency as well as through alliances with our federal partners and with numerous stakeholder organizations.
Janet Woodcock, M.D.
Director, Center for Drug Evaluation and Research
Drug Safety Oversight Across the Product Lifecycle
A key component of the FDA mission is to protect the public health by assuring the safety of human drug products. Supporting this objective, FDA is also responsible for advancing public health by facilitating the scientific innovations that can help make medicines safer while assuring that the public receives clear, accurate, science-based drug safety messages.
The Food, Drug and Cosmetic Act defines drugs, in part, by their intended use, as "articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease" and "articles (other than food) intended to affect the structure or any function of the body of man or other animals".
This report uses the terms “drug” and “drug product” to refer to prescription and over-the-counter (OTC) medicines in their finished, FDA-approved and marketed form. A drug product’s finished form is often a single drug, but may include two drugs manufactured in combination. A drug product may have different routes of administration, such as oral (tablets and capsules), liquid solutions and suspensions, or injection.
In 2006, recognizing that the complexities of drug safety oversight had entered a new and challenging era, FDA requested that the Institute of Medicine evaluate the Agency’s existing drug safety system.
The Institute’s study published in 2007, noted a range of concerns, including ambiguous legislative authorities and a lack of reliable postmarket data about the risks and benefits of drugs. These and other barriers limited FDA's capacity to assess the safety of drugs once they entered the marketplace, or, at times, to take specific regulatory actions when needed. Responding to the IOM’s recommendations, the Agency identified three primary areas of focus that would operate in tandem to support a broad-based drug product safety program:
• Drug Safety Science. Strengthening the science that supports the medical product safety system at every stage of the product life cycle, from premarket testing and development through postmarketing surveillance and risk management.
• Operations and Management. Improving operations and management to ensure implementation of the review, analytical, and communication processes needed to strengthen the U.S. drug safety system.
• Safety Communications. Expanding and improving communication and information flow among all stakeholders engaged in promoting the safe use of medical products.
These focal areas, as they continue to guide and inform FDA’s drug safety enterprise, function in tandem in leveraging assets and delivering benefits through contributions from across the Agency.
The central responsibility for managing, overseeing, and coordinating drug safety efforts rests with the Center for Drug Evaluation and Research (CDER). CDER evaluates new drugs before they can be marketed and maintains a rigorous postmarketing safety surveillance program, monitoring the use of marketed drugs for unexpected health risks (see ‘Postmarketing Safety Surveillance and Oversight: MedWatch, FAERS and the Sentinel System’ below).
CDER also implements safety oversight by regulating the manufacture of drugs and by setting standards for drug quality, and holds numerous public meetings to facilitate expert and consumer input on pending drug safety decisions and priorities.
CDER’s assessment of a drug’s safety profile begins early in the drug development process—long before a product is approved for marketing. A pharmaceutical company or other “sponsor” that is developing a new drug will have first invested several years in laboratory research and animal studies. If early data supports further product development, a sponsor may elect to submit an Investigational New Drug Application (IND) to CDER. An IND will include results from animal studies, toxicity data (information about side effects that cause harm), drug manufacturing data, and plans for human studies to be conducted (clinical trials).
The “sponsor” of a drug product’s development is typically a pharmaceutical company, but can also be a medical institution, university, foundation, a Federal research agency such as the National Institutes of Health, or even (rarely) an individual physician or researcher.
Collaboration and consultation supports and enhances drug product safety from the time an IND is submitted. CDER assigns a multidisciplinary review team to an IND, comprised of project management and scientific review staff, including physicians, pharmacologists, toxicologists, chemists, and statisticians. This team has 30 days to review an IND submission, a process that not only assesses interim findings to determine their acceptability, but protects volunteers who will participate in clinical trials from unreasonable and significant risk. Although an IND can be placed on a “clinical hold” for various reasons, it is usually approved if the technical data is acceptable and proposed clinical trials meet Federal standards.
If the resulting evidence from early development (laboratory tests and animal studies), and all human clinical trials support a drug’s safety and efficacy for its intended use, a company can file a New Drug Application (NDA) to market the drug.
An expanded multidisciplinary team is formed at this point, including staff with expertise in epidemiology, drug surveillance, and risk management. These review teams evaluate the available information about a drug’s safety and efficacy to make determinations about whether the drug can be marketed for use—but also to identify what additional activities, if any, a sponsor must perform in the postmarketing period to further ensure the safety of the drug.
Drug approval decisions are based on a comprehensive assessment of the benefits of the drug and its known and potential risks. In some cases, these decisions are informed by discussions with CDER’s external expert advisors, such as an Advisory Committee or the Drug Safety Oversight Board, which provide input on uniquely challenging drug issues.
The Safety First Initiative, launched in 2008, provides CDER’s overarching framework for identifying, assessing and acting upon drug safety issues in the postmarket period.
"The agency continually evaluates the safety of drug products, and CDER's ongoing commitment to rigorous and continued drug safety evaluation in the postmarket period is reflected in its Safety First Initiative—which outlines CDER's policies and procedures to ensure that equal attention is given to postmarket drug safety as is given during premarket drug review." – Mwango Kashoki, MD, MPH, Associate Director for Safety, Office of New Drugs, CDER
Safety First in Action
Through an amalgam of internal policies and organizational procedures, Safety First’s primary objective is to support and reinforce drug safety by bringing equal focus and attention to the performance of new drugs after they are on the market as is given during premarket review, helping improve the safe use of drugs throughout their lifecycle. Safety First directs that multidisciplinary teams within CDER assess postmarket drug safety signals as they arise, identify appropriate actions for the Center and/or pharmaceutical companies to take, and monitor those actions. All these activities have become integral aspects of CDER’s overall drug safety program.
In mandating and supporting team-based multidisciplinary collaborations, Safety First supports the alignment of safety processes and policies and serves as a foundation for CDER's safety initiatives, programs, and projects. Based on safety data collected during development of a new drug or new safety information identified after a drug is approved and marketed, CDER may find that additional studies are needed to better characterize a drug safety concern and may require further studies as a condition of continued marketing approval. CDER’s review teams may also consider whether a Risk Evaluation and Mitigation Strategy (REMS) is necessary. (A REMS is a safety strategy to manage a known or potential serious risk associated with a medicine, to ensure that its benefits outweigh its risks, and to enable patients to have continued access to the medicine by managing its safe use.)
Postmarketing safety oversight always involves multidisciplinary and collaborative assessment of reports of unwanted or dangerous side effects associated with the use of specific drug products (known as “adverse events”), reported medication errors, and findings from REMS and postmarketing studies. Based on safety information that emerges during the postmarket period—particularly as a new drug is used among more people for longer periods of time—CDER may determine there is an identified risk that alters the benefit-risk balance of the drug and may make regulatory decisions to minimize or manage that risk. The nature of these decisions will vary based on the impact of the risk, and can include safety labeling changes (see “Safety Labeling Changes (SLCs): New and Improved Access to Safety Information” below), additional safety studies, a new or modified REMS, or withdrawal of a drug from the market.
In providing the operational structure for evaluating and acting on drug safety issues in the postmarket period, Safety First supports and reinforces safety oversight across the product lifecycle and serves as an integral aspect of CDER’s overall drug safety program.
Advancing Drug Safety Science
Drug safety science, which includes the development of tools, techniques, and data sources that help CDER to better identify and manage safety issues related to drug effects and product quality, is an integral component of CDER’s regulatory science portfolio. As profound new insights into disease processes at cellular and molecular levels operate in the technologically advanced environments of distributed digital networks, data mining, custom-built software platforms, and mobile device applications, drug safety science is taking on ever more powerful potential to predict, detect, and prevent drug-related adverse events.
JumpStart – Enhancing Premarket Drug Review with Digital Tools
Drug applications submitted in electronic form are easier for CDER to store and manage than paper-based submissions, which can exceed many thousands of pages. CDER has encouraged drug companies to submit INDs and NDAs in electronic format for over 10 years. (Electronic submission of most INDs and NDAs will be required by May 2017.)
As pharmaceutical developers and manufacturers submit electronic product applications in ever greater numbers, the applications have also grown in complexity. In turn, CDER’s workload has increased, and digital tools used to analyze large amounts of data are critical to consistent, reliable, and expeditious assessments of drug safety and efficacy.
The scientists and physicians who serve on CDER’s review teams, although experts in their respective specialty areas, may have difficulties and spend valuable time navigating the digital intricacies of electronic drug product submissions. JumpStart, a vital new service in CDER’s portfolio, is meeting this challenge.
Created by CDER’s Office of Computational Science (OCS), housed within the Office of Translational Sciences (OTS), JumpStart was recognized as an Health and Human Services (HHS) Secretary’s Pick for the 2014 HHS Innovates program, for advancing the modernization of the drug review process. JumpStart represents a first step in the creation of a modern integrated review environment that supports FDA’s 21st Century regulatory review goals.
JumpStart is a software platform that provides an early assessment of data quality and product safety to CDER’s review staff, enabling reviewers to spend less time manipulating complex data and more time applying their expertise to identify safety and efficacy concerns and support informed decision making.
JumpStart Updates—2015-2016
JumpStart has been offered to CDER’s drug review teams for the third year, providing reviewers with better understanding of submission data earlier in the review process, highlighting areas of potential concern and facilitating the efficiency of safety signal identification and risk analysis.
- Thirty full service JumpStart training sessions were provided for drug reviewers. Based on feedback, the JumpStart team improved the data fitness sessions to better meet the needs of reviewers.
- Work proceeded on assessing the feasibility of integrating clinical trials data with JumpStart. The Janus Clinical Trials Repository (CTR) is a data warehouse that can provide access to study data in ways that reviewers need, whether as an individual study or a set of studies integrated for safety assessments. OCS also explored the use of CTR to facilitate analyses of safety risk across classes of drugs or focused on specific treatment indications.
- A robust training program continues to ensure that newly developed digital tools are seamlessly adopted across the review community to improve premarket safety assessments.
More computing tools have been incorporated into the JumpStart service—combining automated analyses for data fitness and safety. Next steps include the piloting of additional tools and services, expanding data fitness assessment capabilities, and building analysis “libraries” to include more analyses aligned with specific areas of drug review.
Postmarketing Safety Surveillance and Oversight: MedWatch, FAERS, and the Sentinel System
After a newly-approved drug enters the marketplace, postmarketing experience can reveal adverse events (AEs) not detected during clinical trials or pre-approval review. FDA maintains two major systems for postmarketing drug surveillance, a “passive” system known as FAERS (FDA Adverse Event Reporting System) and an “active” system known as the Sentinel System.
FAERS – FDA Adverse Event Reporting System
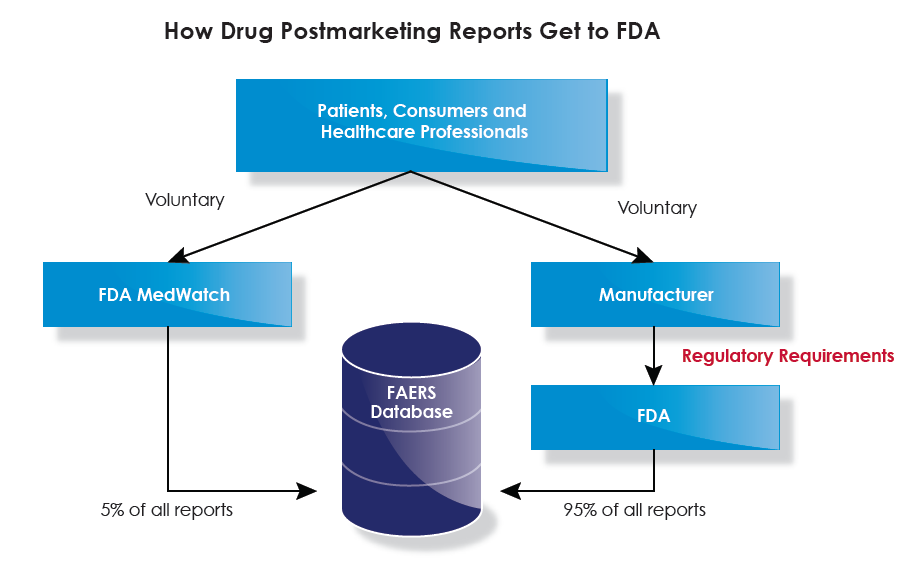
One key tool employed to characterize and evaluate adverse effects (AEs) is the FDA Adverse Event Reporting System (FAERS), an electronic data base of spontaneously submitted adverse events (AEs) associated with drugs and biologic products. For the past 47 years, spontaneous reporting has been the cornerstone of CDER’s postmarketing drug safety monitoring, based on voluntary MedWatch reports from the public, healthcare professionals, and others, of AEs, drug quality problems, or medication errors they observe during the use of a marketed drug product.
MedWatch is FDA’s system for the reporting and collection of clinically important safety information about marketed human medical products.
About 95 percent of the 1.4 million MedWatch reports received annually come from drug product sponsors or manufacturers; the remainder come directly from the public. The objective of CDER’s review of spontaneous reports is to identify serious, rare, or unexpected AEs in order to spot “safety signals”—indicators of new or unanticipated drug safety concerns. The public can use a variety of methods to submit reports, including electronically, on paper, via phone calls or via fax.
FAERS helps to bridge the gap between known AEs seen in pre-approval clinical trials and those seen once an approved drug is used in the general population. If a potential safety concern is identified in FAERS, further evaluation is conducted on a systematic basis. Monitoring of individual spontaneous reports and aggregate data from the FAERS database for all marketed products is conducted weekly. Reviews of AE reports and benefit-risk evaluation reports occur at quarterly, bi-annual and annual intervals, and full drug product safety reviews are conducted 18 months after approval. CDER employs data mining techniques to efficiently identify FAERS reports of greatest value, and is continuously working to update these approaches.
When reviewing reports, particular attention is paid to those AEs that are both serious and unexpected. An important part of the review focuses on the narrative submitted by the person reporting the AE. The more complete a report’s medical and clinical information is, the more useful the report will be, and reporters are encouraged to include as much detail as possible in their narratives.
When a safety concern is detected, further evaluation might include studies conducted using other large databases such as those housed in the Sentinel System, the Centers for Medicare and Medicaid Services, and the Veterans Health Administration, among others. Depending on the results of additional evaluation, CDER may take regulatory action to improve product safety and protect the public health, such as updating a product’s labeling information, called a safety labeling change, communicating new safety information to the public, or, in rare cases, removing a product from the market.
Even though CDER receives more than one million AE reports every year, not all AEs associated with a given drug are reported. Many factors influence whether or not an event will be reported, such as the time a product has been on the market or the nature of publicity about a drug-related event. Because AEs are substantially underreported, FAERS data cannot be used to calculate the incidence of a specific AE or other problem in the U.S. population. It is also understood that reports submitted to FAERS are a highly selective sample of the events observed in real-world medical practice, which are frequently confounded by factors such as multiple drug use and worsening of pre-existing disease. As a result, it is rarely, if ever, certain that a reported AE was actually caused by an associated drug product. CDER does not require that a causal relationship between a product and event be proven before reporting, and reports may not always contain enough detail to properly evaluate the event. Despite these limitations, FAERS remains a very useful surveillance tool that CDER is actively working to improve.
In 2016 alone, the FAERS database will have logged nearly two million AE reports, and now contains more than 12 million reports.
The graph below shows the number of direct, 15-day, and non-expedited (also known as periodic) reports received and entered into FAERS from 2004 through 2015. FAERS receives direct reports from the public while 15-day and non-expedited reports are submitted by industry. The 15-day reports describe AEs from spontaneous reports that are serious and unexpected, as well as AEs from clinical trials that are serious, unexpected and judged to be reasonably associated with the drug. Industry submits all other adverse event reports as non-expedited reports.
The Sentinel System
The Sentinel System is a national, integrated electronic system for monitoring drug safety through shared health care databases (with care taken to protect personal health information). Sentinel leverages “big data” and broad networks across many data partners to systematically detect and respond to emerging risks associated with FDA-regulated products—allowing evaluation of safety issues more rapidly than has been possible in the past. As an active digital surveillance infrastructure focused on recognizing and characterizing drug safety issues, Sentinel enables FDA to proactively assess medical product safety under real-world conditions and thus complements existing postmarketing monitoring capabilities, including FAERS. In the words of CDER Director Janet Woodcock, “FAERS is an invaluable asset, and we’re not seeking to replace it. However, the Sentinel System offers the exciting possibility of not waiting for safety information to come to us in the form of reports, but rather enables us to go out and get that information, adding greatly to our safety monitoring capability. This is active surveillance.”
An important foundation of the Sentinel System is its collaborative nature and active engagement with a broad range of stakeholders, including academic medical centers, healthcare systems, health insurers, and patient advocacy groups. With data analytics leveraged across broad digital networks, and with access to information provided by data partners, including electronic health record systems (EHRs), administrative and insurance claims databases, and registries, Sentinel enables FDA to obtain vital data to help evaluate safety issues—and demonstrates the benefits that can be achieved through broad collaboration.
In addition to its vital contribution to drug safety surveillance, the Sentinel System is also an invaluable national scientific resource for a broad range of other applications. A current goal is that, working with other scientific groups, a National Data Infrastructure can be created that enables many users (such as other governmental agencies or researchers from academia or industry) to access various types of digital data for various purposes, including evaluating medical product performance and healthcare quality improvement.
In 2015-2016, Sentinel’s pilot project, “Mini-Sentinel”, transitioned to a fully up and running Sentinel System. Other milestones in this time period include:
- The Active Risk Identification and Analysis (ARIA) System was integrated into FDA’s day-to-day regulatory and review activities. A subcomponent of the Sentinel System, ARIA leverages existing analysis methods and Sentinel’s Common Data Model to evaluate safety signals identified during both premarketing and postmarketing settings.
- CDER used Sentinel’s rapid query capability in ARIA to run nearly 2,000 different medical product scenarios, and to access the electronic healthcare data (with privacy controls in place) of over 193 million health plan members.
- CDER continued to coordinate with the Center for Biologics Evaluation and Research to expand their vaccine and blood products surveillance capabilities under their Postmarket Rapid Immunization Safety Monitoring (PRISM) and Blood Surveillance Continuous Active Network (BloodSCAN), and with the Center for Devices and Radiological Health to complement the National Evaluation System for Medical Devices (NESMD).
- Blue Cross/Blue Shield of Massachusetts and the Hospital Corporation of America (HCA) came aboard as Sentinel data partners. HCA provides access to data from inpatient hospitalizations, including inpatient medications and transfusions, expanding Sentinel’s reach and capabilities.
Improving Drug Safety through Research: The Safety Research Interest Group and Division of Applied Regulatory Science
Safety Research Interest Group
Since the 2011 report Identifying CDER’s Science and Research Needs was developed by CDER’s Science Prioritization and Review Committee, activities to identify regulatory science and research needs and priorities have continued. CDER is engaged in many drug safety-related regulatory science efforts as well as activities by CDER’s Safety Research Interest Group (SRIG) to identify priority gaps that could be addressed through targeted research projects. These priorities are assessed within the broad framework of FDA’s overall regulatory science objectives and evolving needs which help guide development of CDER’s science and research agenda.
After developing and implementing a process to help guide priorities for safety-related research needs, CDER’s SRIG identified seven major areas of safety-related needs:
- Improve access to postmarket data sources and explore the feasibility of their use in analyzing safety signals.
- Improve risk assessment and management strategies to reinforce the safe use of drugs.
- Evaluate the effectiveness of risk communications of drug safety information to health care providers and the public.
- Improve product quality and design, manufacturing processes, and product performance relating to safety.
- Develop and improve predictive models of safety in humans.
- Improve clinical trial statistical analyses for safety, including benefit-risk assessments.
- Investigate clinical biomarkers of safety, including standards for biomarker qualification.
A “biomarker” is a factor that can be objectively measured and serves as an indicator of normal or abnormal biological processes or responses to a therapeutic intervention such as taking a medicine or receiving a treatment.
Biomarkers can help to confirm a diagnosis, predict a likely medical outcome, or monitor a drug’s effectiveness, among other uses. Familiar examples are measuring blood pressure to measure the effectiveness of a blood pressure medication, or blood sugar levels to measure the effectiveness of a diabetes medication. In these cases, blood pressure measurement or blood sugar level are biomarkers. Common biomarkers also include liver function studies, cholesterol levels, or an electrocardiogram.
Other biomarkers are more complex, such as specific genes or proteins that may be linked to specific or rare conditions. Biomarkers can also be used in the drug development process for a variety of purposes including monitoring drug safety during the investigational period, or identifying the optimal dose of a medicine.
The SRIG reviewed research activities within each of the seven research needs, focusing on potential public health and regulatory impact, feasibility, and the need for CDER involvement or external collaboration or resources. To connect priority projects to resources, the SRIG met with relevant CDER staff to better understand and discuss the scope of needed research. Selected current and ongoing safety-related research projects include:
Electronic health care data to better determine risk of suicide related to use of approved drug products. In recent years there have been regulatory actions with various drugs and drug classes involving increased risk of suicide related to use of those drugs. CDER must enhance its ability to determine if suicide is a potential adverse event of a specific drug therapy. However, suicidal outcomes are infrequent and difficult to measure in clinical trials. Electronic healthcare database studies can measure outcomes for large populations at lower costs than other forms of data-gathering, but their ability to accurately measure suicidal outcomes is uncertain. CDER investigators are therefore conducting a systematic review to investigate which data resources and methods have been most informative for determining suicidal outcomes in populations of interest. Available data are being assessed for their suitability to determine suicide as an outcome of drug therapy, and possibilities for enhancing future data collection on completed and attempted suicide will be explored.
Consumer reactions to drug product labeling. This research focuses on generating systematically collected and robust information on consumer reactions to drug labeling, to inform ongoing regulatory action and future guidance development around over-the-counter (OTC) proprietary (brand) drug naming and the front label panel of a drug product’s box or packaging (the “principal display panel presentation”).
Computer modeling to better predict adverse drug events. Innovative approaches in computer science are being explored to better predict drug-related adverse reactions, by integrating medical and laboratory data with other data based on current medical knowledge and published research. These tools aid in understanding timing and association of drug safety signals with specific enzymes following certain anticancer treatments. As a result, investigators developed novel approaches such as machine learning and neural networks (in which computers can “learn” as new data is collected) to aid regulatory agencies, industry, academia and others to understand adverse events and guide drug development and regulatory reviews.
Identification of factors explaining new safety signals in pre- and postmarket settings. This project will identify factors that might explain the emergence of drug safety issues in the pre- and postmarket settings—and the extent to which scientific evidence, along with approaches used in precision or “personalized” medicine (where drugs or treatments are tailored to an individual based on that person’s genetic make-up and other personal factors) can be used to predict and manage safety issues. This project will compile a “catalog” of past decisions made by CDER to place a drug approval on hold or when postmarketing safety label changes were needed. Researchers plan to map that data to possible risk management strategies, which can assist drug product reviewers and safety surveillance as well as regulatory decision-making in the postmarketing period.
Division of Applied Regulatory Science
Science evolves rapidly—and CDER must stay abreast of that evolution to maintain 21st Century drug review and safety surveillance capacities. In the Division of Applied Regulatory Science (DARS), researchers using state-of-the-art equipment and technologies are applying a wide range of expertise in areas such as toxicology, pharmacology, biophysics, chemistry, genomics, and computational modeling, in the service of developing new and innovative approaches that will improve CDER’s drug product review capabilities.
DARS scientists provide:
- Improved lab assays for predicting drug safety
- Better understanding of the mechanisms underlying drug toxicity
- Insights into the links between a drug’s chemical structure and its potential to induce adverse events.
- Research-based predictions that can be used to support decisions for new and generic drugs when safety data are limited.
DARS conducts studies that cannot or will not be done by industry or other Federal agencies, with a staff that provides technical capabilities using a range of tools to predict such safety-related outcomes as genotoxicity, carcinogenicity, cardiotoxicity and drug-induced liver injury. DARS consultations assist CDER safety reviewers in the interpretation of data submitted in INDs and NDAs—which are often followed by new research conducted to address knowledge gaps in key areas.
DARS scientists are currently involved in over 30 projects that advance drug development and safety assessment to help inform regulatory decision making.
- Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis (SJS/TENS) are serious and potentially life-threatening skin reactions that can occur in individuals treated with a variety of drugs, and are therefore of significant concern to CDER and the larger medical community. To better understand underlying mechanisms, DARS researchers conducted analyses that identified a specific protein as a significant mediator of SJS/TEN. Leveraging a genomics database, DARS found that the drug carbamazepine disrupted several genes, including one associated with carbamazepine-induced SJS/TEN. This information that can be applied in future drug reviews as well as postmarket safety oversight.
- While autopsy findings can identify cardiac impairment, they cannot specify if impairments were caused by drug products. DARS uses a number of laboratory and computer-based approaches to measure the impact of specific sets of drugs on heart function. These combined approaches will allow better prediction of both the functional effects and scope of injury associated with cardiotoxic drugs, thereby enhancing the safer use of medications.
- In another effort to investigate drug-induced cardiac toxicity, DARS is part of a research consortium of worldwide regulatory agencies, industry, and academic centers evaluating the possibility of predicting drug-induced cardiac toxicity using a computer model of the human ventricular (heart muscle) cell. This project involves evaluating drugs and then simulating their effects on electrical activity in the heart under a wide range of conditions. The study seeks to develop a new regulatory pathway that will change current cardiovascular safety testing guidelines and potentially eliminate the need for certain kinds of clinical testing during drug development.
- DARS is pursuing development of industry standards for biomarker discovery and evaluation, including gene-based research methods and reference data sets, to develop consistent criteria to measure tissue damage in pathology samples.
- DARS is evaluating digital tools that may help to predict and explain drug-related adverse events. These tools can be used to generate possible explanations for why an adverse event might occur, and have the potential to help modernize drug safety analyses, to improve product labeling, and guide development in the premarket phase as well as supporting pharmacovigilance in the postmarketing period.
- DARS is evaluating a software program that generates adverse event profiles based on a drug’s molecular targets in the human body, to predict adverse events not previously seen in clinical trials and therefore not included in the original product label. Predictions of drug-related adverse events have already been made using this approach. Planning is underway to perform similar analyses on many more drugs now under review.
DARS research is tightly linked to current and emerging drug product reviews and is customized to answer specific questions. The value of DARS’ resident expertise to CDER regulatory science is enhanced through targeted collaborations across FDA and with industry and academia, providing additional opportunities to build on its past research successes and improve the way drugs are developed and reviewed.
Advancing Drug Safety Science through Public-Private Partnerships
Drug safety science—the development of methods, tools, and data sources employed to better identify, predict, assess, and manage safety issues related to drug products—is an essential component of CDER’s regulatory science portfolio. Creating the cutting-edge scientific knowledge base needed to support 21st Century safety science requires an understanding of the knowledge gaps in product safety, the resources that can be leveraged to address those gaps, and the necessary expertise of internal and external partners.
This effort cannot be done alone but rather requires partnerships between FDA and multi-sector stakeholders sharing the common goal of promoting drug safety and efficacy to protect and improve public health.
The Critical Path Initiative (CPI), launched in 2004, is a keystone initiative to forge stakeholder collaborations to tackle various drug safety and drug development issues.
These strategic partnerships help to enhance the efficiency and quality of medical product development, evaluation, and manufacture through scientific advances—the “critical path”. When a new or growing public health concern is identified that cannot be effectively addressed by a single organization, CDER and other elements in FDA, operating within the framework of the CPI, can ask a non-profit stakeholder organization to serve as a neutral convener that can then leverage the expertise of numerous other stakeholders through a public-private partnership (PPP).
PPP stakeholders may include industry, academic institutions, patient advocacy groups, government agencies, and others.
PPPs come together pre-competitively to provide a platform to bring new insights to regulatory and drug safety science questions. CDER staff members currently serve as liaisons to several PPPs, providing a regulatory perspective and assisting with the development of public health objectives, which may take various forms including white papers, registries, databases, disease models, and biomarker development. The products of PPP efforts are shared in the public domain for all stakeholders with the goal of streamlining drug development, increasing the efficiency of regulatory decision-making, and enhancing public health.
Some examples of drug safety goals and objectives from current and ongoing CDER-facilitated PPPs are outlined below.
Predictive Safety Testing Consortium (PSTC). PSTC’s mission is to scientifically validate or “qualify” new biomarkers for the detection and monitoring of drug-induced toxicities (side effects). To date, PSTC has achieved qualification for kidney injury biomarkers seen in rats, and PSTC members are currently engaged in discussions with CDER and its European counterpart, the European Medicines Agency, to qualify clinical (tested in humans) safety biomarkers for drug-induced liver, muscle, cardiac and blood circulatory system, kidney, testicular, and pancreatic injury. PSTC’s efforts on additional kidney and muscle injury biomarkers have been recognized for their public health potential by CDER through Letters of Support, which enhance the visibility of PSTC’s work, encourage data sharing, and stimulate additional studies for further evaluation of these biomarkers. Once such safety biomarkers are developed and well understood, they can be used widely during drug development to make risk/benefit decisions—both by regulators who will have fresh insights into the safety implications of new drugs, and later by patients and health care providers who can make more informed health decisions regarding the potential consequences of taking a drug.
International Serious Adverse Event Consortium (iSAEC). The iSAEC mission is to identify variations in human genes that predict the risk of drug-related serious adverse events. Some patients are genetically predisposed to certain responses to drugs, including certain side effects. The iSAEC’s research examines the impact of genetic variation on how individuals respond to a large variety of medicines. iSAEC’s initial studies have successfully identified genetic variants associated with drug-related liver toxicity and serious skin rashes. Through better understanding of the genetic basis for such adverse events in patients, informed health decisions can be made regarding an individual patient’s predisposition to the risk of taking specific medications.
Cardiac Safety Research Consortium (CSRC) and Health and Environmental Sciences Institute (HESI) Cardiac Safety Technical Committee. CSRC is advancing scientific knowledge on cardiac safety for new and existing drug products by building a collaborative research environment based on the principles of the CPI as well as other public health priorities. The HESI Cardiac Safety Technical Committee seeks to improve public health by reducing unanticipated drug-related cardiovascular adverse effects and developing new approaches for early detection and prediction of cardiovascular side effects. The potential for new drugs to cause heart rhythm changes (known as arrhythmias) is one of the batteries of safety tests performed during drug development. Currently, this safety evaluation of drug-induced arrhythmias is measured through changes seen in an electrocardiogram. CDER is collaborating with CSRC and HESI’s Cardiac Safety Technical Committee to develop an innovative laboratory test that can serve as a much improved drug toxicity assessment of new drugs.
Through the expertise and resources of external scientists working in the PPP model, vital new information is shared in a pre-competitive space that is defining the path forward for drug safety and drug development science. Through the products of such partnerships, CDER is better positioned to ensure that new drugs are safe and effective prior to marketing, to continue to assess and monitor safety once drugs are on the market, and to help patients and health care providers make better decisions about the drugs they use.
Improving Operations and Management in Support of Drug Safety
CDER has undertaken a number of program and policy enhancements over the last 18 months, enlisting Center operational and management initiatives in the implementation of new or revised policies. These include internal reorganizations that maximize CDER’s rich human capital resources and better align those resources on behalf of a state-of-the-art drug quality and safety program. As new challenges emerge and are addressed—including concerns related to tracking of postmarketing drug safety issues and studies and the call for a rigorous response to the national opioid abuse and addiction crisis—CDER continues its focus on reducing preventable harms from medication use, enhancing drug product quality efforts, and improving oversight of generic drugs, compounded drugs, and drug supply chain security.
The 2015 GAO Report: A Call To Improve Data on Safety Issues
CDER monitors and reviews safety information about all drugs throughout their lifecycles, interacting with pharmaceutical companies during product development and clinical investigation of the drug, closely reviewing safety issues during consideration for marketing approval, and, if approved, monitoring safety reports after the drugs are marketed. After approval, CDER may learn of new, more serious, or more frequent adverse drug reactions from, for example, post-approval voluntary or mandatory reporting of adverse drug reactions during use of the drug, post-approval clinical trials exploring new uses of the drug, or other post-approval studies including active surveillance evaluations.
CDER integrates information from these sources with its own evaluations into an overall system of postmarket surveillance and risk assessment, and uses this aggregated information when a drug’s identified risks indicate a need to undertake any of a number of actions. These actions can include providing additional safety information to the public, updating drug labeling, requiring postmarket studies or trials or other risk management interventions, and, rarely, withdrawing approval of a drug.
When there is an unmet need for the treatment of a serious medical condition, CDER may use one or more of its expedited programs, including the “fast track” or “breakthrough therapy” designations, intended to bring drugs to market and expedite their availability to patients. CDER is also responsible for monitoring the safety of these marketed drugs and reporting on those efforts.
In December 2015, the Government Accountability Office (GAO) issued a report responding to a Congressional request that GAO provide information about aspects of CDER’s postmarket monitoring activities.
GAO was tasked with providing information on CDER’s expedited programs and its postmarket monitoring of expedited and nonexpedited drugs. GAO’s report examined the number and types of requests for fast track or breakthrough therapy designation that CDER received, and the number and types of FDA-approved drug applications that used an expedited program, focusing on “the extent to which FDA’s data on tracked safety issues and postmarket studies allowed the agency to meet its reporting and oversight responsibilities.”
GAO analyzed CDER data on requests for fast track or breakthrough therapy designation and approved drug applications that used an expedited program between October 1, 2006 and December 31, 2014. GAO also interviewed relevant CDER staff and reviewed CDER information on tracked safety issues and postmarket studies, concluding that:
- “FDA lacks reliable, readily accessible data on tracked safety issues and postmarket studies needed to meet certain postmarket safety reporting responsibilities and to conduct systematic oversight.”
- “While internal control standards for the federal government specify that information should be recorded in a form and within a time frame that enables staff to carry out their responsibilities, and that relevant, reliable, and timely information should be available for external reporting purposes,” GAO found that CDER’s “data on postmarket safety issues and studies were incomplete, outdated, contained inaccuracies, and was stored in a manner that made routine, systematic analysis difficult.”
GAO acknowledged that CDER has supported efforts to shorten development and streamline review of drug applications through expedited pathways, but noted problems with “efforts to oversee and track potential safety issues and postmarket studies once those drugs are on the market.”
In responding to the GAO report, CDER clarified that, to be approved, all drugs must meet the same standards for safety and effectiveness—whether or not a particular drug has been the subject of an expedited review program. CDER also noted that, based on continuous improvement processes launched prior to the initiation of the GAO study, the challenges related to administrative tracking of the two aspects of the Center’s postmarket safety work noted above had already been recognized and remedies were underway. Consistent with GAO’s recommendations, CDER is continuing to address those issues through ongoing improvement efforts.
- TSIs: Using an internal database, CDER tracks postmarket drug safety issues to ensure their timely evaluation and management. These issues are referred to as tracked safety issues, or TSIs.
- PMRs: CDER may also require drug companies to conduct postmarketing studies or clinical trials to further characterize known or potential serious risks of a drug. These studies and clinical trials are referred to as postmarketing requirements, or PMRs.
- PMCs: Drug companies frequently recognize the importance of certain other postmarketing studies and, at FDA's request, commit to conducting those studies. A postmarketing study that is agreed to, but not required, is called a postmarketing commitment, or PMC.
Postmarketing Requirements and Commitments
CDER is continuing with efforts to improve its ability to maintain accurate and timely data on the status of PMRs and PMCs. A recent internal audit of CDER data, which began in 2014 and continued into 2015 (and was ongoing at the start of and during GAO’s investigation), resulted in multiple data updates and corrections, allowing the Center to implement additional oversight mechanisms to ensure the timeliness and accuracy of PMR and PMC data.
CDER’s most recent annual assessment found that most open PMRs and PMCs are progressing on schedule.
Tracked Safety Issues
TSIs are one of the many ways in which information about significant safety issues is shared within CDER and across the Agency, joining the larger system of safety oversight programs and resources described in this report. At the time the GAO report issued, CDER had already noted that its administrative tracking of TSIs needed re-evaluation and updating. The Center recognized that the overall number of TSIs entered into the system established for tracking safety issues was low compared to the number of postmarketing safety issues already identified and, therefore, TSIs were not being effectively recorded in the database in a timely manner. CDER addressed this issue and continues to do so: As of June 30, 2016, 154 new TSIs have been created and 171 retroactive TSIs have been added to the database. New TSIs are being entered into the tracking system immediately or shortly after they are identified.
Optimizing Drug Safety Oversight
CDER needs every available tool in its efforts to maintain a robust, versatile, and technologically optimized drug safety oversight program. In response to the GAO report and based on CDER’s own program assessments, the Center continues to implement new processes and procedures to correct data and address gaps in information. CDER is also engaged in activities to increase and streamline the capture of specific information into its database and is facilitating additional analyses for oversight of postmarket studies and safety issues—reflections of the Center’s commitment to completing implementation of these and other new safety monitoring processes and procedures in a timely manner.
Opioid Addiction and Abuse: Addressing a National Crisis
Another kind of drug safety issue emerges when drug products are misused—not used as directed, or used inappropriately after being prescribed. This is the case with the national opioid abuse and addiction crisis. The nation is experiencing a devastating epidemic of prescription opioid misuse and abuse, including a large number of overdose deaths.
Rates of opioid use and abuse began to accelerate in the late 1990s and continued to chart a steep increase over the next 15 years. While the reasons for this phenomenon are varied and complex, three key facts illustrate the breadth and seriousness of the crisis:
- According to the Centers for Disease Control and Prevention, 259 million prescriptions were written for opioids in 2012—more than enough to provide every American adult with a prescription.
- The 2014 National Survey on Drug Use and Health reported that an estimated 4.3 million people aged 12 or older were current nonmedical users of pain relievers.
- The National Center for Health Statistics identified drug overdose as the leading cause of accidental death in the United States with 18,893 overdose deaths related to prescription pain relievers as of the end of 2014.
Department of Health and Human Services statistics tell us that on an average day in the U.S., more than 650,000 opioid prescriptions are dispensed, 3,900 people initiate nonmedical use of prescription opioids, and 78 people die from an opioid-related overdose.
As this public health problem has grown in scope, FDA has taken a number of significant actions aimed at doing our part to address it. Consistent with the Comprehensive Addiction and Recovery Act of 2016, signed by The President on July 22, the Agency is committed to using all available tools to continue its focus on the following goals:
- Improve the safe use of opioids through careful and appropriate regulatory activities and policy development, and through communication, partnership and public discussion.
- Improve the treatment of pain through improved science.
As a part of this work, FDA recently developed a comprehensive action plan to take concrete steps toward reducing the impact of opioid abuse on American families and communities. A pivotal element of this plan commits many elements within CDER and the Agency to seek outside input, including advice from Advisory Committees on many opioid-related issues. At the same time, FDA is committed to re-examining the risk-benefit paradigm for opioids and to ensure that the Agency is considering the wider public health effects of its decisions regarding opioids.
Actions that FDA is committed to taking include the following:
Expanded use of Advisory Committees. The FDA will convene an expert Advisory Committee before approving any new drug application for an opioid that does not have abuse-deterrent properties. Additionally, the Pediatric Advisory Committee will offer expert advice regarding a framework for pediatric opioid labeling before any new labeling is approved. The FDA will also convene an Advisory Committee on abuse-deterrent formulation (ADF) opioids when those medicines raise unexpected issues.
Development of warnings and safety information for immediate-release (IR) opioid analgesic labeling. In order to better inform doctors about the risks of opioid analgesics and how to prescribe them safely, FDA is requiring changes to IR opioid analgesic labeling to include prominent warnings and safety information. These safety labeling changes incorporate elements similar to the extended-release/long-acting (ER/LA) opioid analgesics labeling changes that were finalized in 2014.
Strengthening of postmarket requirements. The scientific evidence needed to guide the use of opioid analgesics—particularly in the setting of long-term use—is substantially lacking. FDA is strengthening requirements for ER/LA opioid analgesic sponsors to conduct postmarket studies and generate data on the long-term impact of using ER/LA opioid analgesics, developing better evidence on the serious risks of misuse and abuse associated with long-term use of opioid analgesics and predictors of opioid addiction.
Updating Risk Evaluation and Mitigation Strategies (REMS). FDA can require a REMS from manufacturers to ensure that the benefits of a drug outweigh its risks. (REMS are safety strategies designed to manage known or potential serious risks associated with a medicine. A REMS may be required as part of the approval of a new product or for an approved product when FDA becomes aware of new safety information.) ER/LA opioid analgesics are currently subject to a REMS program that requires drug companies to fund professional medical education on the appropriate use of these products, which they must offer to health care providers at low or no cost. FDA will update the REMS program requirements for ER/LA opioid analgesics after considering Advisory Committee recommendations, with a goal of increasing the number of prescribers who receive training on pain management and safe and appropriate prescribing of ER/LA opioid analgesics.
Expanded development and use of effective abuse-deterrent formulations (ADFs) to discourage abuse. The pharmaceutical industry has shown significant interest in developing ADFs as the technology to do so has continued to rapidly progress. ADFs hold promise as their abuse-deterrent qualities continue to improve and as they become more widely available. CDER has recently issued draft guidance with its recommendations for the approval standards for generic abuse-deterrent formulations. Release of this guidance is a high priority, as the availability of less costly generic products should accelerate prescriptions written for ADFs, while also fueling innovation and generic ADF product development. We are also working to assess the real-world impact of these new formulations to make certain they are having the positive effects on abuse we anticipate based on the premarket testing.
Support for better treatments for pain and for opioid overdose. Naloxone is a drug used to counteract the effects of opioid overdose. CDER recently approved naloxone in an intranasal form (administered by spraying into the nose). Building on this approval, CDER is reviewing options, including over-the-counter availability, to make naloxone more available. While CDER continues its assessment, the Agency actively supports the Centers for Disease Control and Prevention guidelines for prescribing opioids for the treatment of pain, and will facilitate the development of evidence and improved treatments to bring broader access to overdose treatment, safer prescribing and use of opioids, and ultimately, new classes of pain medicines without the same risks as opioids.
Reassessment of the risk-benefit approval framework for opioid use. CDER received advice from the Agency’s Science Board in March 2016, and has engaged the National Academies of Sciences, Engineering, and Medicine on how to take into account our evolving understanding of the risks of opioids—not only for patients but also the risks of misuse by other persons who obtain them. Both activities will generate publicly available reports serving to incorporate the broader public health impacts of opioid abuse in FDA’s drug product approval decisions.
Each of these action items is keyed to specific outcomes, such as:
- Facilitating opportunities for expert advice from external medical and scientific authorities, and for public input before approval of any new opioid that does not have abuse-deterrent properties
- Developing, and providing doctors with, better information about the risks of opioids and how to prescribe these drugs safely
- Improving scientific evidence to guide the use of opioid medications, particularly in long-term use settings, by strengthening requirements for drug companies to generate postmarket data on the long-term impact of using extended-release/long-acting opioids
Safe Use Initiative
As many as 1.5 million Americans are injured or killed each year by inappropriate use of FDA-regulated drugs, including intentional or accidental misuse, or errors such as receiving the wrong drug or wrong dose from a pharmacy or doctor. Many of these deaths or injuries are preventable.
As an external public outreach complement to Safety First, CDER instituted the Safe Use Initiative (SUI) to develop solutions within the broader healthcare environment that go beyond standard regulatory methods to enhance the safe and appropriate use of drugs for all Americans. SUI seeks to reduce preventable harm from medications by developing, implementing, and evaluating cross-sector interventions with partners committed to safe and appropriate medication use. As part of this long-term initiative, SUI brings together various parts of the health care system to develop interventions that measurably reduce preventable harm from medications.
Selected 2015-2016 program updates include:
Opioid Patient-Prescriber Agreement. SUI’s facilitation of open collaboration with a multidisciplinary group of federal and non-federal stakeholders was launched in 2012 and continued through 2015 and into 2016. Collaborators include addiction specialists, pain management specialists, primary care physicians, dentists, pharmacists, medical malpractice insurer, third party insurers, patients previously addicted to opioids, and caregivers of patients taking opioids. The working group’s goal is to develop a model opioid patient-prescriber agreement (PPA) designed to be a shared decision-making tool regarding pain treatment as well as a mechanism to support discussion of critical information about opioids. The Opioid PPA was piloted for acceptability, feasibility and usability with 14 prescribers and 117 patients. Data analysis has been completed and a report describing the results of the pilot is currently in development.
Multi-state Prescription Drug Monitoring Program Surveillance Database. In 2015 the SUI team partnered with the Bureau of Justice Assistance and Brandeis University to develop a surveillance database using state Prescription Drug Monitoring Programs that will enable CDER to identify opioid use patterns.
Post-Surgical Opioid Intervention. Kaiser Foundation Research Institute and the Kaiser Permanente Center for Health Research, with support from the SUI, are developing a prediction tool to identify patients at high risk for opioid use following surgery, adapting an opioid-sparing educational intervention and estimating the efficacy of the intervention in a randomized controlled trial. (Enrollment underway.)
Development of a risk score for severe hypoglycemic events. SUI partnered with Kaiser Permanente to provide health care providers with a practical way to identify patients at high risk of severe hypoglycemia and guide shared decision-making with respect to modifications of glucose-lowering therapy in out-patient clinical settings. The prediction model has been completed and validation is underway.
Healthy People 2020 – Medical Product Safety Topic Area Working Group. Healthy People provides science-based, 10-year national objectives for improving the health of all Americans. SUI team members are leading the Medical Product Safety Topic Area Working Group as part of the HHS Healthy People 2020 Initiative. This group is revising an Initiative developmental objective focused on reducing deaths from the use of pain medicines.
Standardizing the preparation of medications to reduce errors. With SUI support, the University of Michigan gathered information on current practices involving preparation of oral liquid medications in pharmacies, and developed voluntary standard concentrations in compounding medications to reduce the risk of errors when patients change pharmacies or transition their care. This study ended May 2015 and received the annual Cheers Award from the Institute for Safe Medication Practices, recognizing superlative standards of excellence in the prevention of medication errors and adverse drug events. One of the investigators is now working on a national standardization effort with SUI and the Association of Health-Systems Pharmacists.
SUI’s ongoing efforts, working across a spectrum of activities to support and facilitate the safe use of prescription medicines, spans federal partnerships, public-private workgroups, collaborations throughout health and medical communities outside of government, the enlistment of experts beyond medical providers and administrators to include insurers, patients, and patient caregivers, as well as the funding of original research to better understand the nature of prescription medicine misuse and errors.
Working to Ensure Drug Product Quality
What can go wrong with drug products after approval, but before and during distribution to pharmacies, clinics, and hospitals across America? Many things, from inherent defects in product design, to failures in drug product manufacturing related to outmoded equipment or aging manufacturing facilities pressed beyond capacity, or inadequate or ineffective on-site quality management systems. In addition to these sorts of direct concerns related to manufacturing problems, production slow-downs or failures can create drug shortages—many of which involve critically needed life-saving medicines used in hospital emergency departments and operating rooms.
The quality of drug products is a pivotal aspect of drug safety, making product quality oversight a vital asset in CDER’s drug safety portfolio. In facilitating needed growth in CDER’s product quality programs, CDER Director Janet Woodcock spearheaded the stand-up of the new CDER Office of Pharmaceutical Quality (OPQ) in January 2015.
CDER’s drug quality oversight is now structured to streamline regulatory processes and align areas of expertise by integrating review, inspection, surveillance, policy, and research activities to strengthen pharmaceutical quality on a global scale.
Supporting this central objective is a systematic approach to product quality knowledge and informatics that brings parity to the oversight of brand name and generic drugs and domestic and international manufacturing facilities, while also promoting emerging technologies to enhance pharmaceutical quality. These efforts are moving toward a reinvigoration of the pharmaceutical manufacturing sector in the United States by, among a number of activities, centralizing regulatory review, policy, research and science functions, project management, quality management systems, and administrative activities, and establishing a uniform approach to pharmaceutical quality across all manufacturing facilities, domestic or foreign, and across all drug product areas—new drugs, generic drugs, and over-the-counter drugs.
These tenets establish “One Quality Voice” for patients, providers, and industry, creating the “critical mass” that ultimately assures the availability of safe, effective, high-quality medicines to the American public.
OPQ recently celebrated its first anniversary, marking several milestones and achievements including:
- Establishment of the CDER Emerging Technology Program featuring a cross-function team that will provide drug companies with opportunities to discuss design and development issues related to their proposed new technology prior to the submission of drug applications. The program also published a draft guidance that will help drive the pharmaceutical industry towards a more efficient, agile, and flexible manufacturing sector.
- Development of a risk communication dashboard that will complement or even possibly replace the existing drug product quality review infrastructure, which is based upon subjective, unstructured and lengthy text narratives.
- Establishment of a risk-based approach in quality assessment to capture knowledge of risks over a product lifecycle. This risk-based approach will enhance quality, transparency and consistency of assessment of drug products and will lead to more efficient and effective regulatory decisions and clear expectations for industry.
- Establishment of new policies to ensure that drug quality activities support the availability of quality medicines for all Americans by developing and clearly communicating science and risk-based policies and standards related to drug product quality, including application review and inspection.
Generic Drug Safety Oversight: A Coordinated Effort
CDER’s Office of Generic Drugs (OGD) established its Clinical Safety and Surveillance Staff (CSSS) in 2014. The CSSS facilitates broad collaborative surveillance projects with various Offices throughout CDER, establishing coordinated partnerships that further CDER’s commitment to the surveillance of generic drug quality and therapeutic equivalence.
The CSSS continues with tracking and evaluation of reports of potentially inferior generic product quality, adverse events and other safety concerns, and reports of different therapeutic effect compared to the relevant reference listed drug (also known as the “brand name” drug). The results of these investigations are further evaluated by an interdisciplinary team and, if a significant safety issue is identified, that team works collaboratively with other relevant CDER offices to resolve the issue.
The interdisciplinary CSSS structure facilitated evaluation of several high profile surveillance efforts, integrating the efforts of all contributors to the CDER Generic Drug Program. A recent example is the evaluation of therapeutic non-equivalence of generic versions of methylphenidate (indicated for treatment of adult attention deficit/hyperactivity disorder and narcolepsy), thorough reviews of formulation differences, bioequivalence criteria, and clinical use of the brand-name product (Concerta), which led to a downgrade of therapeutic equivalence ratings for two generic versions, a multidisciplinary workgroup recommendation for changes to the bioequivalence criteria, and a major change to the product-specific bioequivalence criteria. The Office of Generic Drug’s Regulatory Science Program also launched a prospective study to further evaluate therapeutic differences between brand-name and generic versions of Concerta.
Compounded Drugs and Drug Supply Chain Security: Critical Aspects of Product Safety
Compounded Drugs
Compounding is a practice in which a licensed pharmacist, a licensed physician, or, in certain cases, a person under the supervision of a licensed pharmacist, combines, mixes, or alters ingredients of a drug to create a medication tailored to the needs of an individual patient. Compounded drugs can serve an important role for patients whose clinical needs cannot be met by an FDA-approved drug product, such as a patient who has an allergy and needs a medication to be made without a certain dye, or an elderly patient or a child who cannot swallow a tablet or capsule and needs a medicine in a liquid dosage form that is not otherwise available. But drugs that are compounded improperly, or that are compounded under substandard conditions, can pose serious health risks. For example, compounded drugs that are produced under insanitary conditions can become contaminated, and errors made during compounding can result in sub- or super-potent products.
CDER has responded to many serious adverse events, including infections and deaths, associated with compounded drugs that were contaminated or otherwise compounded improperly. The most serious in a long history of outbreaks associated with contaminated compounded drugs was an outbreak of fungal meningitis in late 2012. Over 750 patients in 20 states were diagnosed with a fungal infection after receiving injections of the contaminated drugs, and 64 patients in nine states died.
Since the 2012 fungal meningitis outbreak, CDER has identified insanitary conditions at many of the compounding facilities it has inspected. Among many other insanitary conditions that put patients at risk, examples include dog beds and dog hairs in close proximity to a sterile compounding room, pharmacies with dead insects in ceilings, renovations made without any evidence of controls to protect sterile drugs from contamination, use of coffee filters to filter particulates, compounding by personnel with exposed skin during sterile production, toaster ovens used for sterilization, and a kitchen dishwasher used to clean sterile compounding equipment and utensils.
Congress enacted the Drug Quality and Security Act (DQSA) in 2013. The DQSA removed certain provisions from section 503A of the Federal Food, Drug, and Cosmetic Act (FDCA) that were found to be unconstitutional and created a new section, 503B, in the FDCA. Under this new section, a compounder can become an “outsourcing facility.” Outsourcing facilities must comply with current good manufacturing practice requirements, are inspected by FDA according to a risk-based schedule, and must meet certain conditions, including reporting adverse events to FDA associated with their compounded drugs.
CDER has worked quickly to implement the compounding provisions of the DQSA, while directing inspection and enforcement initiatives to protect the public from poor quality compounded drugs. In 2015 and to date in 2016, CDER:
- Continued inspection and enforcement efforts, as well as policy development, related to human drug compounding.
- Directed and evaluated findings from over 110 inspections of compounders throughout the United States – with problems identified in a majority of inspections, prompting numerous compounders to initiate voluntary product recalls and, in some cases, to stop sterile production.
- Issued more than 35 warning letters to compounders. In addition, in 2015, two compounders entered into consent decrees of permanent injunction (a legal agreement to permanently comply with FDA regulatory policies).
CDER has also continued to make significant progress in implementing the compounding provisions of the FDCA by publishing policy documents applicable to compounding pharmacies and outsourcing facilities. In 2015 and so far in 2016, CDER has published twelve draft guidance documents, four final guidances, and a draft memorandum of understanding with the states, and also held the first five meetings of the reconstituted Pharmacy Compounding Advisory Committee.
As problems are identified at compounding pharmacies and outsourcing facilities across the country, CDER will continue to vigorously pursue inspection and enforcement efforts.
In late 2015, CDER Director Janet Woodcock discussed drug compounding in a podcast, offering an overview of the process, risks, and benefits.
Drug Supply Chain Security
The U.S. drug supply chain remains one of the safest in the world—but has also become increasingly complex as the supply chain reaches beyond U.S. borders. Threats to the supply chain such as counterfeiting, theft, and importation of unapproved or otherwise substandard drugs, could result in unsafe and ineffective drugs in U.S. distribution.
FDA has been actively engaged in efforts to improve the security of the drug supply chain for many years to protect patients from unsafe, ineffective, and poor quality drugs. Since the formation of the first FDA Counterfeit Drug Task Force in 2003, the Agency has advocated for a multi-tiered approach to securing the supply chain and protecting consumers from the threats posed by counterfeit or compromised drug products. The ability to trace prescription drugs also plays a significant role in providing transparency and accountability in the drug supply chain.
The Drug Supply Chain Security Act (DSCSA) was signed into law in 2013, providing for a uniform Federal framework to be in place by 2023. This framework will identify and trace certain prescription drugs by outlining critical steps to build an enhanced electronic, interoperable system to trace drug products as they are distributed within the United States. This critically important system expands and improves FDA’s ability to protect the public from exposure to drugs that may be counterfeit, stolen, tainted, or otherwise potentially harmful.
Since January 1, 2015, the law placed new requirements on drug manufacturers, repackagers, and wholesale distributors, followed by dispensers (primarily pharmacies), to have systems and processes in place to exchange product tracing information, conduct verification to identify suspect product, including steps such as quarantining and investigating suspect product, notify FDA and trading partners when an illegitimate product is found, and keep records.
CDER has issued several guidances for industry and continues to work with stakeholders on implementation through public workshops and other meetings. To help inform the enhanced system of 2023, CDER is coordinating with members of the supply chain and developing a pilot project program that will explore and evaluate methods to enhance the safety and security of the pharmaceutical distribution supply chain. This pilot project program will focus on utilization of the product identifier, which will enable product tracing down to the level of an individual package.
Communicating Drug Safety: A Global Public Interface
As CDER’s preeminent resource for communicating human drug information, the Center’s Office of Communications (OCOMM) serves at the nexus of virtually every drug safety issue, question, or crisis that CDER faces. With nearly 100 staff members including health professionals, communications specialists and web and graphic designers, OCOMM fulfills CDER’s internal and external communication needs, including providing strategic communication advice to Center and Agency leadership, developing and coordinating overarching public communication initiatives and educational activities, and employing comprehensive communication approaches to ensure consistent branding, messaging, and strategic direction of CDER’s communication products—all critical aspects of drug product safety.
Expert Responses to Public Queries
A team of pharmacists, consumer safety officers and nurses respond to public inquiries from around the world, providing responses to questions and expert guidance regarding human drug products.
More than 65,000 requests for information are received annually, many of which involve some aspect of drug safety, from how much or how little of a medicine to safely use, to safe prescribing or dispensing practices, to responses on media-trending drug safety concerns. Queries arrive via phone, electronic mail, and letter, from an array of stakeholders, including consumers, health care professionals, academia, foundations and research organizations, and the pharmaceutical industry. Safety inquiries can involve complex scientific or regulatory issues; in those cases OCOMM coordinates extensive research and consultation needs with the subject matter experts needed to craft accurate and balanced responses.
Another variation in public inquiry are national write-in campaigns, which are frequently organized around a particular facet of medication safety. OCOMM organizes and manages CDER’s response to these campaigns—in the last 18 months alone, OCOMM has sent over 2,000 emails and letters to 16 different campaigns.
Social Media and Online Tools
OCOMM serves as CDER’s public interface in using multiple digital tools and platforms to provide drug safety outreach and education. The highlights below reflect 2015-2016 milestones in these communications arenas.
Facebook. CDER became the first Center to engage the public on FDA’s Facebook page, with 128 drug-related posts reaching over 4 million Facebook users.
Twitter. CDER became the first FDA Center with a Twitter account (@FDA_Drug_Info). 616 Tweets were sent, and 40,764 new followers were gained over the last 18 months to reach a total of more than 200,000 Twitter followers. OCOMM organized and led the first CDER Twitter Chat, #LungChat, which engaged major oncology organizations, patients, advocates and health care professionals about lung cancer treatments, and live-tweeted three of Center Director Janet Woodcock’s recent Congressional testimonies.
Podcasts. FDA Drug Safety Podcasts provide Drug Safety Communications in audio form and are available on iTunes and ReachMD radio—46 Drug Safety Podcasts have seen 31,846 transcript visits and 135,388 audio downloads in 2015 and through the first quarter of 2016. The Director’s Corner is an audio podcast series featuring Dr. Janet Woodcock commenting on a number of CDER drug safety issues and advances, including:
- Celebrating 30 years at FDA. Dr. Woodcock reflects on breakthroughs in drug research and regulation and makes some predictions for the future of drug products.
- Talking Translational Science. Dr. Woodcock defines translational science and how it can positively affect drug development and review.
- Looking Back and Moving Forward in 2016. Dr. Woodcock discusses major events of 2015 and priorities for 2016.
- Drug Compounding. Dr. Woodcock discusses drug compounding from both a safety and regulatory standpoint.
- Working with Patient Advocacy Groups. Dr. Woodcock discusses regulatory guidances drafted and submitted to FDA by patient advocacy groups.
Webinars. OCOMM hosted 27 webinars over 2015 and to date in 2016, several of which offered information and training for several drug safety programs and initiatives:
- Introduction to Postmarketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER
- Introduction to FDA’s MedWatch Adverse Reporting Program
- Professional Labeling: The Prescribing Information
- FDA Online Drug Information Resources for Students and Clinicians
- Introducing the REMS@FDA Website
- Know Your Source: Protecting Patients from Unsafe Drugs
Video. FDA Drug Info Rounds Videos target pharmacists and other health care providers and are promoted to State Boards of Pharmacy, pharmacy schools, and posted on YouTube. Many of the Drug Info Rounds Videos produced in 2015 and 2016 focus on aspects of drug safety.
- REMS@FDA Tutorial presents the new and improved REMS website called REMS@FDA.
- Emergency Preparedness – Keeping Medications Safe discusses the importance of having a plan in place for keeping medications safe as part of emergency preparedness.
- MedWatch Tips and Tools presents resources available to health care professionals to make reporting to MedWatch easier than ever.
- Breakthrough Therapy discusses the breakthrough therapy designation, an exciting new program to expedite drug development while maintaining safety oversight in the development process (Bronze Telly Award).
- FAERS provides background information about the FDA Adverse Event Reporting System (FAERS) database.
- REMS presents the many components of Risk Evaluation and Mitigation Strategies (REMS) and how they can help manage a drug product with known or potential serious risks.
- Disposal of Unused Medicines discusses safe medication disposal options.
Drug Information Update. The FDA Drug Information Update email subscription service sends bulletins to stakeholders with safety information about drug products including Drug Safety Communications, drug approvals, CDER Statements, and industry guidances. The Drug Information Update email service gained 28,999 new subscribers in 2015 (with a current total of 155,447), and the service sent out 208 email messages.
Drug Safety Communications (DSCs)
Patients, caregivers, health care professionals, and the public are updated on new drug safety information with Drug Safety Communications (DSCs). These messages and announcements can range from new or emerging risks associated with concurrent conditions (for example, pregnancy or existing liver or kidney disease), or cautions about potential medication errors. DSCs contain actionable recommendations for patients and health care professionals that can help them make more informed decisions, and prevent or mitigate drug-related harm.
DSCs are usually issued in conjunction with regulatory actions such as Safety Labeling Changes for a number of reasons, including issues affecting a large number of patients, potentially serious or life-threatening adverse events, or medication errors that may result in serious or life-threatening adverse reactions.
The Drug Safety Communication web page is the most visited page on the FDA’s web site. Forty-six DSCs were issued between January 1, 2015, and July 26, 2016, and during that time the DSC home page and the 46 individual DSC web pages received over a million page views. Outreach was significantly greater than that, however, as DSCs are much more broadly circulated through a variety of other channels, including several large listservs, email newsletters, podcasts, social and traditional media, and targeted outreach to media, healthcare professionals, advocacy groups and other stakeholders.
Safety Labeling Changes (SLCs): New and Improved Access to Safety Information
When a drug is approved for marketing, not every safety concern or risk potential can be identified. As this report highlights, postmarket safety oversight is therefore essential to learning more about the effects of a medicine when used by a large number of people over a long period of time. If new safety concerns emerge after a medicine is used in a real-world setting, CDER may require a “Safety Labeling Change,” or SLC. These changes can focus in one or more specific sections of a drug label, including contraindications, warnings and precautions, adverse reactions, or boxed warnings (widely known as “black box warnings”).
The “labeling” of a medicine is the detailed information on the package insert that accompanies a drug, be it inside the product box or folded and glued to the lid of a bottle—not simply the information printed on a bottle or box. The product labelingl includes a summary for the safe and effective use of the drug and is generally intended for use by prescribers and pharmacists. FDA-approved patient information is written for a lay audience and is generally included at the end of the product label.
Although SLCs have been available online for many years, they were aggregated and posted on a monthly basis. This meant that if a new safety concern was reflected in an approved labeling change early in a month, that information was not publicly posted until early in the following month—three to four weeks later. As web and database technologies evolved, CDER recognized that it needed to apply new digital functionalities that would not only shorten the time between an SLC’s approval and when its safety information becomes available on the FDA’s website, but to generally enhance an online visitor’s experience by improving the SLC web platform’s clarity, access, and navigability.
As of August 1, 2016, CDER’s SLC data and web access functions migrated to a new platform and database managed by OCOMM, making drug safety labeling information available in real time and in a user-friendly format for relevant stakeholders, including health IT and information vendors, health care providers, and patients. Searchability along with ease in downloading data, whether for one drug or several, are both features that can now be used on the newly designed SLC Web platform.
As health IT vendors are able to gather SLCs more efficiently, they can more effectively organize and distribute data related to those SLCs. In turn, this furthers CDER’s primary goal in re-designing the SLC database and Web interface: To deliver drug safety label changes to health care providers as quickly as possible. In turn, this facilitates “downstream” data integration into drug information systems, and will carry SLC data into other communication venues such as social media, news feeds, and web sites with vast reach among health care professionals, patients, and consumers, including WebMD, Medscape, PDR.net, Epocrates, FirstDataBank, and Yahoo Health.
The new CDER website database will be populated with SLCs from January 2016 forward. Data from earlier than January 2016 will continue to be available on the MedWatch website. The table below details the number of SLCs approved between January 2015 and July 2016—more than 1,500 in all.
| BOXED WARNINGS | 87 |
|---|---|
| CONTRAINDICATIONS | 83 |
| WARNINGS | 452 |
| PRECAUTIONS | 548 |
| ADVERSE REACTIONS | 362 |
| PPI / MG / PCI | 144 |
| TOTAL | 1,676 |
Risk Communications Research
OCOMM houses a number of ongoing research studies that will allow better understanding of CDER audiences’ knowledge, perceptions, needs, desires, and behaviors related to a variety of drug safety information. Evidence now being generated will provide data that can be used to improve CDER communications and expand dissemination of content and materials in order to help target audiences understand the health and safety information that CDER provides. These research efforts also provide the public, including people with limited health literacy or who face disparities in accessing health services, opportunities for input on the effectiveness of CDER drug safety information.
In late 2015, a study of medical countermeasures messaging (launched in 2012) was completed, offering insight and recommendations for developing effective communication initiatives for public health emergencies. A second phase of this study will be undertaken in 2016 to test the various medical countermeasures messages that were developed as a result of the initial study findings.
Other OCOMM risk communication research conducted in 2015 and through early 2016 included:
- A study to enhance FDA communications addressing opioids and other potentially addictive pain medications, scheduled to be completed in 2017
- Research exploring issues of uncertainty and unintended consequences associated with prescription drug communications
- A survey project building on prior focus group research that tests Drug Safety Communications to better understand consumer recall, understanding of risk, and behavioral intentions related to DSC content and formatting
Conclusion
The work highlighted in this report—projects, initiatives, partnerships, and original research—is not a complete list of CDER’s safety programs, but represents many of the most dynamic efforts to reinvent and advance the Center’s drug safety enterprise. CDER’s drug safety work capitalizes on team-oriented and multidisciplinary methodologies, tapping synergies within the FDA while also reaching outside the Agency to partner with industry, academia, independent research institutions, and advocacy organizations in the drive to reach critical safety objectives.
As with all areas of science and technology, drug safety science is constantly evolving. CDER recognizes that ongoing success in safety oversight and surveillance requires a continuing ability to adapt to new information, new technologies, and new developments. CDER has created a safety system that directs scientific expertise and rigor across the lifecycle of FDA-approved drugs, building the Agency’s ability to navigate the inevitable changes to come in medicine and healthcare.
Moving forward, all CDER’s safety efforts, as they remain thorough and systematic, will continue to be designed to support parallel efforts to advance innovation and help ensure that safe and effective new therapies are available to the American public as efficiently and rapidly as possible.