FDA Releases Annual Summary Report on Antimicrobials Sold or Distributed in 2022 for Use in Food-Producing Animals
December 7, 2023
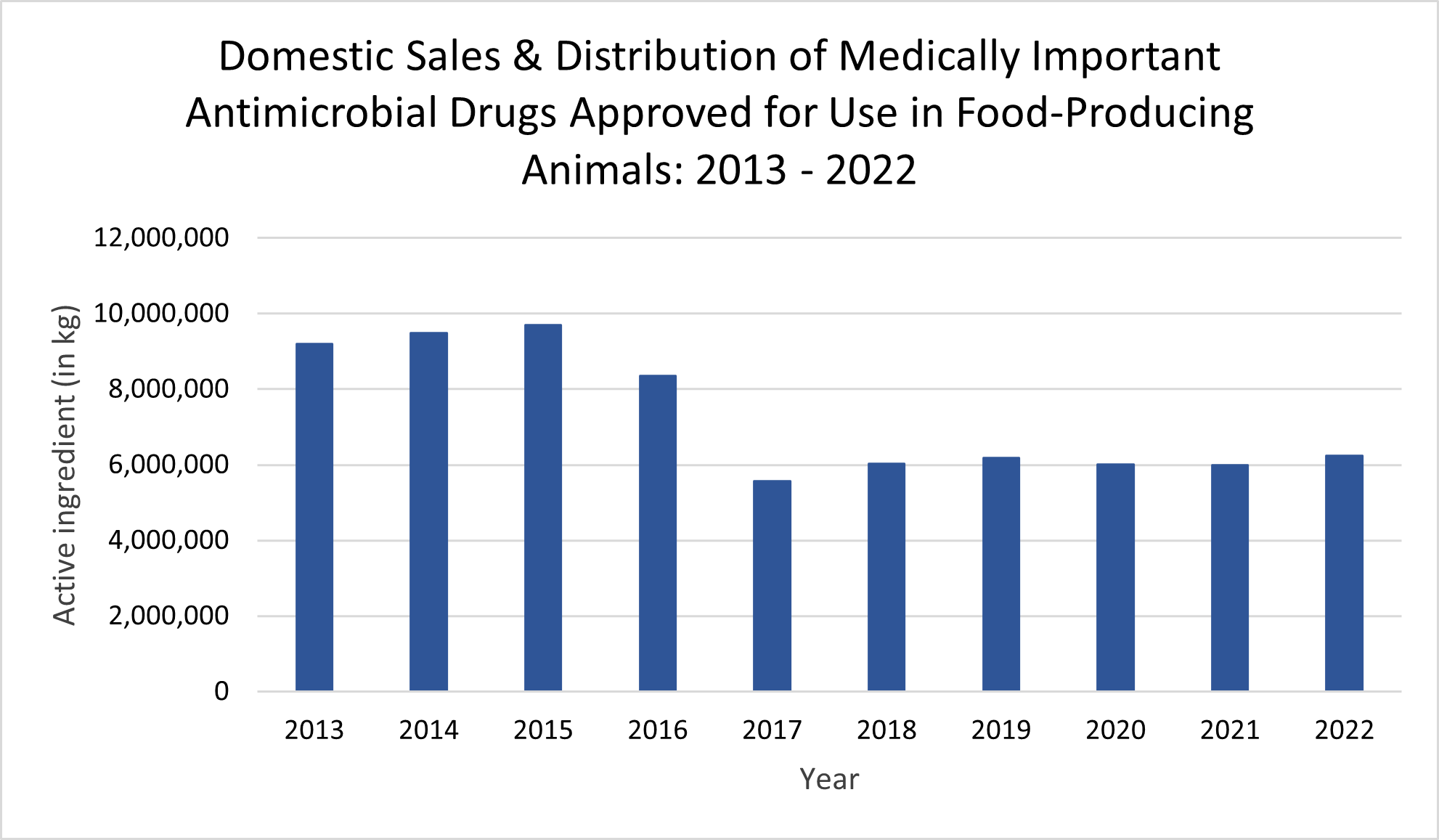
Today, the U.S. Food and Drug Administration’s Center for Veterinary Medicine published the 2022 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. This year’s data show that domestic sales and distribution of medically important antimicrobial drugs approved for use in food-producing animals increased by four percent between 2021 and 2022; compared to 2015 (peak year of sales), 2022 sales were down 36 percent. Sales volume may fluctuate over time in response to various factors, including changing animal health needs or changes in animal populations.
The 2022 data are published in a new dashboard reporting format that allows users to interact with the data and create data visualizations using criteria such as antimicrobial drug class, species, and year. The annual sales data are also available in an Excel spreadsheet. The FDA intends to continue this approach for annual sales and distribution summary reports in the future.
While sales data on antimicrobial drug products intended for food-producing animals do not necessarily reflect the actual use of antimicrobial drugs, sales volume observed over time can be a valuable indicator of market trends related to these products. However, when evaluating the progress of ongoing antimicrobial stewardship in veterinary settings, it is important consider additional information sources including actual use data, animal demographics, animal health data, and data on antimicrobial resistance.
The FDA has also updated its Interactive Summary of Biomass-Adjusted Antimicrobial Sales Data to include the 2022 biomass-adjusted sales data. A biomass denominator adjusts annual antimicrobial sales data to account for the size of the population of a given livestock species in the U.S. potentially being treated with those drugs.
For sustainable and long-term progress in the fight against antimicrobial resistance, the agency continues to foster good antimicrobial stewardship practices and promote the judicious use of antimicrobials so uses are limited to only when necessary to treat, control, or prevent disease. You can learn about CVM’s AMR goals and action items for the next five years from CVM’s five-year plan, “Supporting Antimicrobial Stewardship in Veterinary Settings, Goals for Fiscal Years 2024-2028.”
For more information about what readers should consider when analyzing the report, please review the FDA’s Questions and Answers: Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals.
Additional Information
- 2022 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals
- Data Spreadsheet: Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals (2013-2022)
- Questions and Answers: Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals
- ADUFA Reports
- Final Rule to Collect Antimicrobial Sales and Distribution Information by Animal Species
- Biomass-Adjusted Antimicrobial Sales and Distribution Data in Food-Producing Animals: Interactive Summary
Issued by FDA Center for Veterinary Medicine.
For questions, Contact CVM.