Accelerating Rare disease Cures (ARC) Program
CDER’s ARC Program | Center for Drug Evaluation and Research
The ARC program is seeking input on the clarity, relevance, and usefulness of the Learning and Education to ADvance and Empower Rare Disease Drug Developers (LEADER 3D) educational materials. Please submit comments through this docket by April 3, 2026.
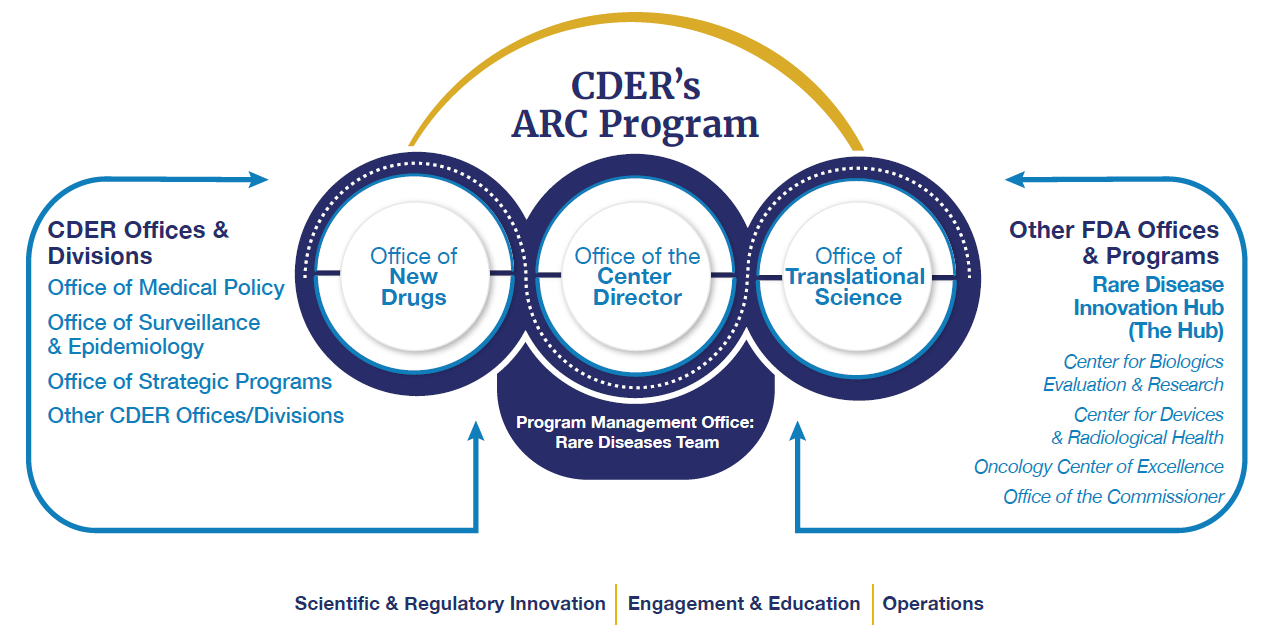
CDER’s Accelerating Rare disease Cures (ARC) Program brings together CDER’s collective expertise and activities to provide strategic overview and coordination of CDER’s rare disease activities. The ARC Program is governed by leadership from across CDER's Office of the Center Director, Office of New Drugs, and the Office of Translational Sciences. The program is managed by CDER's Rare Diseases Team.
Connect with us!
Subscribe to the ARC Newsletter
More About the Accelerating Rare Disease Cures Program
Resources for Rare Disease Drug Development

Rare Disease Drug Development Guidances
Review, by topic, select guidance documents that are relevant to rare disease drug development

LEADER 3D Educational Initiative
Learning & Education to Advance & Empower Rare Disease Drug Developers (LEADER 3D) resources

START Pilot Program Information
Learn about the Support for Clinical Trials Advancing Rare Disease Therapeutics (START) Pilot Program

RDEA Pilot program Information
Rare Disease Endpoint Advancement (RDEA) Pilot program supports novel efficacy endpoint development

Rare Disease Funding Opportunities
Learn about available funding opportunities for rare disease product development

Rare Disease Cures Accelerator
Find efforts to support innovation and quality in rare disease drug development
What's Happening?
Rare Disease News and Events
Learn about the ARC Program’s latest rare disease news, events, and happenings

Rare Disease Drug Approvals
Learn about some of the most recent and notable rare disease drug approvals

ACT for ALS Overview and Updates
FDA’s Initiatives for Rare Neurodegenerative Diseases including Amyotrophic Lateral Sclerosis (ALS)
Lysosomal Diseases C-Path Pre-Consortium
A partnership to address unmet needs for those living with these diseases and how to get involved