Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/P) Inspection Information
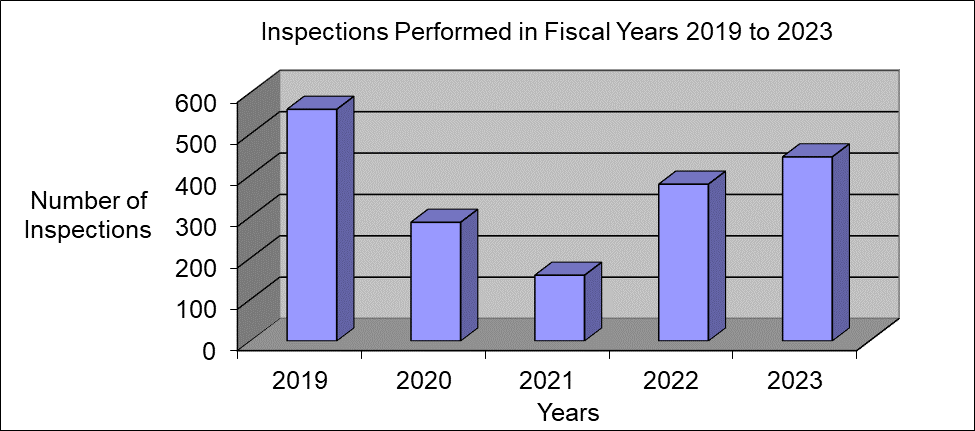
Inspections Performed in Fiscal Years 2019 to 2023
Inspection Conclusion by Fiscal Year
| 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|
| Inspections Classified NAI | 440 | 236 | 111 | 295 | 350 |
| Inspections Classified VAI | 111 | 40 | 44 | 73 | 93 |
| Inspections Classified OAI | 12 | 8 | 4 | 11 | 2 |
| Number of Inspections1 | 563 | 287 | 159 | 379 | 445 |
NAI = No Action Indicated, meaning no objectionable conditions or practices were found during the inspection (or the significance of the documented objectionable conditions found does not justify further action).
VAI = Voluntary Action Indicated, meaning objectionable conditions were found and documented but the agency is not prepared to take or recommend regulatory action.
OAI = Official Action Indicated, meaning objectionable conditions were found and regulatory action should be recommended.
[1]Sum of inspection classifications does not equal total number of inspections performed by fiscal year due to data constraints.