COMPANY ANNOUNCEMENT
Hospira, Inc. Issues a Voluntary Nationwide Recall for 4.2% Sodium Bicarbonate Injection, USP and 1% and 2% Lidocaine HCl Injection, USP Due to the Potential for Presence of Glass Particulate Matter
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionPotential Presence of Glass Particulates
- Company Name:
- Hospira, Inc.
- Brand Name:
-
Brand Name(s)Hospira
- Product Description:
-
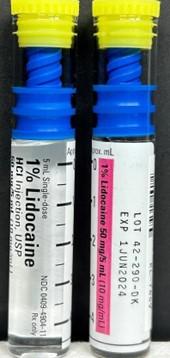
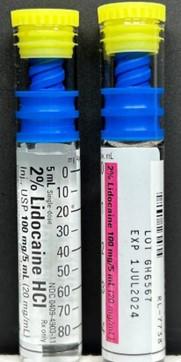
Product Description4.2% Sodium Bicarbonate Injection, USP, 1% Lidocaine HCl Injection, USP, and 2% Lidocaine HCl Injection, USP
Company Announcement
FOR IMMEDIATE RELEASE - October 2, 2023 - NEW YORK, NY., Hospira, Inc., a Pfizer company, is voluntarily recalling the lots listed in the table below of 4.2% Sodium Bicarbonate Injection, USP, 5 mEq/10mL vial; 1% Lidocaine HCl Injection, USP, 50 mg/5mL vial; and 2% Lidocaine HCl Injection, USP, 100 mg/5mL vial to the user level. The recall was initiated due to the potential for presence of glass particulate matter.
There is an unlikely probability for serious adverse events, including death, should a patient receive an injectable product found to contain particulate matter identified as glass. Potential complications related to injection of visible and subvisible inert particles include inflammation of a vein, granuloma, and blockage of blood vessels or life-threatening blood clot events. The frequency and severity of these adverse events could vary depending upon a variety of factors including the size and number of particles in the drug product, patient comorbidities (such as age, compromised organ function), and presence or absence of vascular anomalies. The risk is reduced by the possibility of detection, as the label contains a clear statement directing the healthcare professional to visually inspect the product for particulate matter and discoloration prior to administration.
To date, Hospira, Inc. has not received reports of any adverse events associated with this issue for these lots.
Sodium Bicarbonate Injection, USP is a sterile, nonpyrogenic, hypertonic solution of sodium bicarbonate (NaHCO3) in water for injection for administration by the intravenous route as an electrolyte replenisher and systemic alkalizer. It is indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or severe dehydration, extracorporeal circulation of blood, cardiac arrest and severe primary lactic acidosis. Sodium bicarbonate is further indicated in the treatment of certain drug intoxications, including barbiturates (where dissociation of the barbiturate-protein complex is desired), in poisoning by salicylates or methyl alcohol and in hemolytic reactions requiring alkalinization of the urine to diminish nephrotoxicity of hemoglobin and its breakdown products. It is also indicated in severe diarrhea which is often accompanied by a significant loss of bicarbonate.
Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of an antiarrhythmic agent administered intravenously by either direct injection or continuous infusion. It is available in various concentrations. It is administered intravenously or intramuscularly and is specifically indicated in the acute management of ventricular arrhythmias such as those occurring in relation to acute myocardial infarction, or during cardiac manipulation, such as cardiac surgery.
The NDC, Lot Number, Expiration Date, and Configuration details for the impacted products are indicated below. The products were distributed nationwide to wholesalers/hospitals/institutions in the United States and Puerto Rico from October 13, 2022 through October 26, 2022.
| Product | NDC | Lot Number | Expiration Date |
Presentation | Configuration/ Count |
|---|---|---|---|---|---|
| 4.2% Sodium Bicarbonate Injection, USP Glass ABBOJECT® Syringe | Carton 0409-5534-24 Case 0409-5534-14 |
GJ5007 | 1AUG2024 | 5 mEq/10mL, (0.5 mEq/mL) |
1 vial and injector/ carton 10 cartons/ bundle Case pack 5 X 10- 10mL |
| 1% Lidocaine HCl Injection, USP LIFESHIELD® Glass ABBOJECT® Syringe | Carton 0409-4904-11 Case 0409-4904-34 |
42290DK | 1JUN2024 | 50 mg/5mL (10 mg/mL) |
1 vial and injector/ carton 10 cartons/ bundle Case pack 5 X 10- 5mL |
| 2% Lidocaine HCl Injection, USP LIFESHIELD® Glass ABBOJECT® Syringe | Carton 0409-4903-11 Case 0409-4903-34 |
GH6567 | 1JUL2024 | 100 mg/5mL (20 mg/mL) |
1 vial and injector/ carton 10 cartons/ bundle Case pack 5 X 10- 5mL |
Pfizer places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process. Pfizer has notified direct consignees by letter to arrange for the return of any recalled product.
Wholesalers, hospitals, institutions, and doctors with an existing inventory of a lot which is being recalled should discontinue use, stop distribution and quarantine the product immediately. If you have further distributed the recalled product, please notify your accounts and/or any additional locations which may have received the recalled product. Hospitals/Institutions should inform Healthcare Professionals in your organization of this recall. For additional assistance, call Sedgwick Inc. at 1-800-805-3093 between the hours of 8 a.m. to 5 p.m. ET, Monday through Friday.
Healthcare Professionals with questions regarding this recall can contact Pfizer using the information below.
| Contact Center |
Contact Information | Area of Support |
|---|---|---|
| Pfizer Medical Information | 1-800-438-1985, option 3 (8am to 7pm ET Monday through Friday) www.pfizermedinfo.com | For medical questions regarding the product |
| Pfizer Drug Safety | 1-800-438-1985, option 1 (24 hours a day; 7 days a week) | To report adverse events and product complaints |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being executed with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Sedgwick Inc.
- 1-800-805-3093
- Media:
- Media Relations
- +1-212-733-1226
- PfizerMediaRelations@pfizer.com