Reuse Tracheostomy Tubes or Switch to Appropriate Alternatives During Shortage: FDA Safety Communication
Date Issued: October 31, 2022
The U.S. Food and Drug Administration (FDA) is alerting patients, caregivers, and health care providers that there is a shortage of tracheostomy tubes, including Bivona tracheostomy tubes manufactured by ICU Medical. A shortage of Bivona tracheostomy tubes is more likely to impact pediatric patients because the supply of alternative tubes with similar functionality may be limited. The FDA is working closely with manufacturers and other stakeholders to help quickly resolve supply challenges and support availability of these critical devices for patients who need them. People who use these tubes, including caregivers and health care providers, should consider the FDA’s recommendations listed below.
Recommendations for Patients and Caregivers
The FDA recommends taking these steps to reduce the number of tracheostomy tubes used for each patient during the shortage:
- Follow the manufacturer’s instructions for cleaning, sanitizing, and reusing tracheostomy tubes for the maximum number of times allowed.
- For example, Bivona tracheostomy tubes may be cleaned, sanitized, and reused as described in A Handbook for the Home Care of Your Child with a Tracheostomy for single-patient use, as stated in the indications for use, up to:
- 5 times for pediatric sizes
- 10 times for adult sizes
- For example, Bivona tracheostomy tubes may be cleaned, sanitized, and reused as described in A Handbook for the Home Care of Your Child with a Tracheostomy for single-patient use, as stated in the indications for use, up to:
- Work with your health care provider and durable medical equipment (DME) supplier to determine if appropriate alternatives, such as other FDA-cleared tracheostomy tubes that may use different raw materials, are available.
Recommendations for Health Care Providers
- Review the Recommendations for Patients and Caregivers.
- Discuss these recommendations with patients who use the affected devices and their caregivers.
- Consider using these recommended conservation strategies in health care settings as well as encouraging their use in home settings.
- Contact your distributor or the manufacturer directly to inquire about current inventory, including if appropriate alternatives, such as other FDA-cleared tracheostomy tubes that may use different raw materials, are available.
Device Description
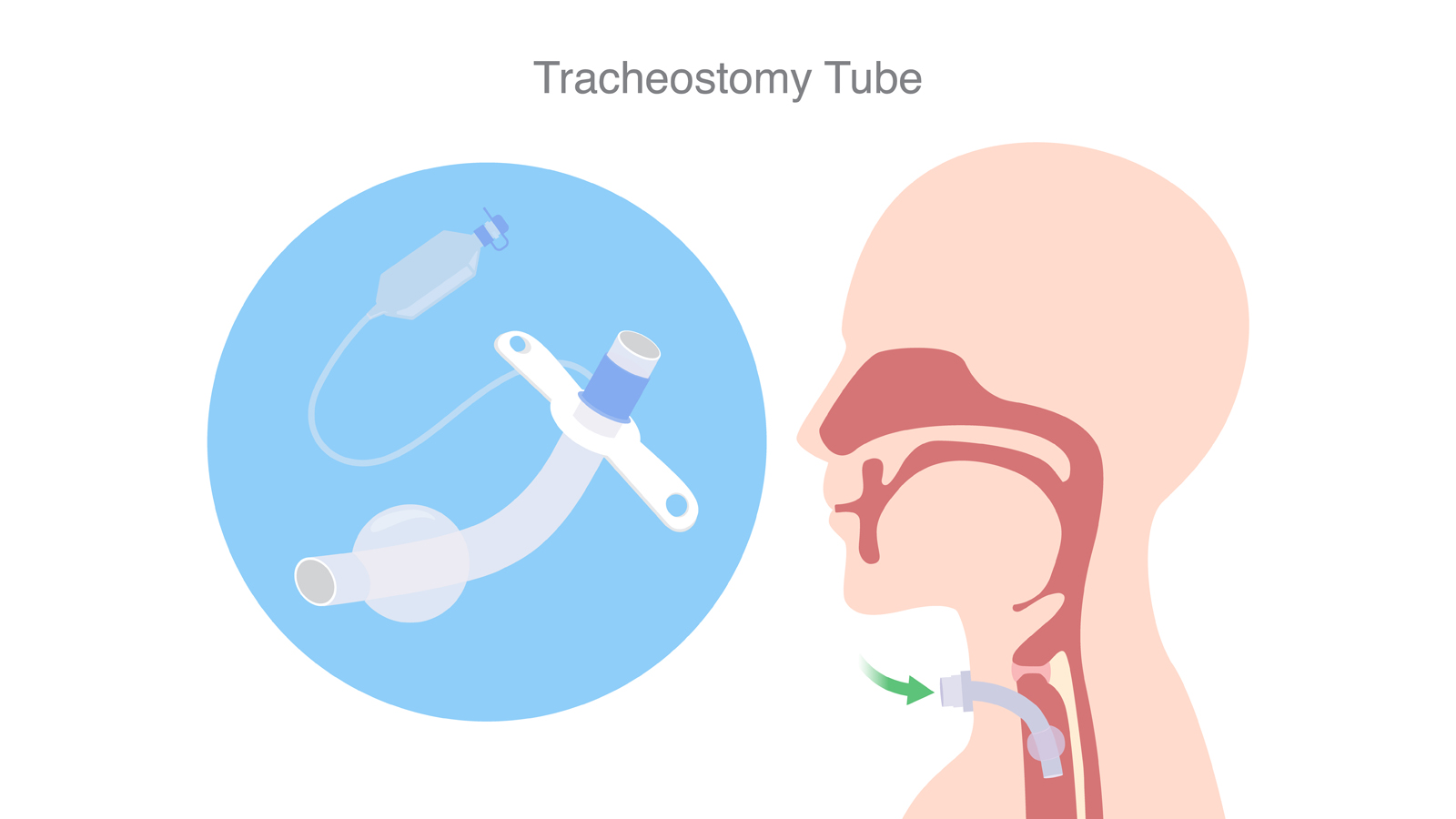
A tracheostomy tube is used to help people breathe following a tracheotomy (a surgical procedure that creates a small opening in the neck directly into the windpipe, or trachea). A tracheostomy tube is placed through the neck opening to allow a patient to breathe through the tube instead of through their mouth or nose. Tracheostomy tubes may be cleaned, sanitized, and reused for single-patient use, as stated in the indications for use.
There are different types of tracheostomy tubes available, and the health care provider will choose the tube that is appropriate for a patient’s needs. The tubes may be used in health care facilities, such as hospitals and long-term care facilities, or at home.
Supply of Raw Materials Unavailable for Certain Tracheostomy Tubes
The FDA is aware that manufacturers have experienced difficulties getting raw materials needed to make these products. The Bivona tracheostomy tube manufactured by ICU Medical is commonly used in pediatric patients because the tube is made from a flexible silicone material which makes them easier to insert in pediatric patients. A shortage of Bivona tracheostomy tubes is more likely to impact pediatric patients because the supply of alternative tubes with similar functionality may be limited. While there are other FDA-cleared tracheostomy tubes for pediatric patients, there may not be enough available to adequately mitigate the shortage. The FDA is aware that ICU Medical has sent communications to customers to provide additional details on supply constraints and efforts to reduce the shortage.
FDA Actions
The FDA is working with manufacturers, Durable Medical Equipment (DME) suppliers, and HHS’s Administration for Strategic Preparedness and Response (ASPR) to help manufacturers obtain the needed raw materials and to help expedite supply of tracheostomy tubes that meet the FDA’s standards for safety and effectiveness.
We recognize the consequences of this shortage on patients, especially pediatric patients who need access to new tubes now. We are working to limit the impact on patients as much as possible by working with the manufacturers and key stakeholders to help ensure availability of the materials needed to make these critical medical devices.
On October 31, 2022, the FDA added tracheostomy tubes to the medical device shortage list (see product code JOH - Tube Tracheostomy And Tube Cuff and product code BTO-Tube, Tracheostomy (w/wo Connector)). The medical device shortage list shows the types of devices the FDA has determined to be in shortage. The FDA will continue to update the list as needed. The FDA also reviews each notification received under section 506J of the Federal Food, Drug, and Cosmetic Act and uses this information, along with additional details about the supply and demand of a device, to determine whether a device is in shortage.
The FDA will keep the public informed if significant new information becomes available.
Reporting Problems with Your Device
If you are experiencing supply issues for tracheostomy tubes or other devices, contact the FDA about a medical device supply chain issue. Reporting supply issues informs the FDA of how it may be able to help address device supply availability.
If you think you had a problem with your device, the FDA encourages you to report the problem through the MedWatch Voluntary Reporting Form.
Health care personnel employed by facilities that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their facilities.
Questions?
If you have questions, email the Division of Industry and Consumer Education (DICE) at DICE@FDA.HHS.GOV or call 800-638-2041 or 301-796-7100.