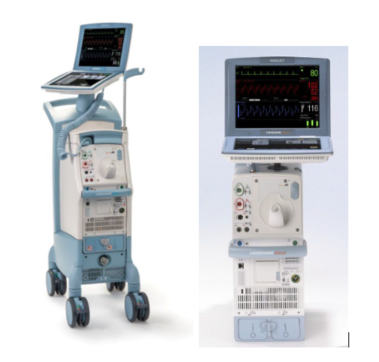
Getinge/Maquet/Datascope Recalls Cardiosave Hybrid and Rescue Intra-aortic Balloon Pumps (IABPs) for Autofill Failure Alarms Resulting in Pump Stops
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
The devices described in this recall are the same devices announced in the UPDATE: Risk of Device Failures for Getinge’s Maquet/Datascope Cardiosave Intra-Aortic Balloon Pump (IABP) – Letter to Health Care Providers on August 31, 2023.
Please be aware, this recall is a voluntary correction, not a product removal.
Recalled Product
- Recalled Product:
- Cardiosave Hybrid Intra-Aortic Balloon Pump (IABP) and Cardiosave Rescue Intra-Aortic Balloon Pump (IABP)
- Product Models:
- Distribution Dates: March 6, 2012 to May 19, 2023
- Devices Distributed in the U.S.: 4586
- Date Initiated by Firm: July 31, 2023
Device Use
The Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pump (IABP) devices are electromechanical systems used to inflate and deflate intra-aortic balloons (IAB). These systems provide temporary support to the left ventricle through counter pulsation. Once the balloon is positioned in the aorta, the pump is set to work in synchrony with the electrocardiogram or arterial pressure waveform to make the balloon inflate and deflate at the right time during the cardiac cycle.
Cardiosave IABPs are indicated for acute coronary syndrome, cardiac and non-cardiac surgery, or complications of heart failure in adults. They are used in health care facilities.
Reason for Recall
Getinge/Maquet/Datascope is recalling their Cardiosave Hybrid and Rescue IABPs because an Autofill Failure alarm may put the device in standby mode resulting in therapy interruption. When IABP therapy begins, the IABP automatically fills the balloon catheter circuit with helium. Every two hours, or during changes to atmospheric pressure, the IABP autofill process occurs. If any step of the autofill process fails or does not complete, the device will generate an “Autofill failure” alarm message and the IABP pump will stop providing therapy. If the alarm persists, the patient may experience a prolonged interruption of therapy, which will require the healthcare provider to obtain a different console to continue therapy. If another IABP console is not available for use, alternative means of providing hemodynamic support (vasopressors, inotropes or alternate therapies) may be initiated by a healthcare provider as a temporizing measure.
Interruption in device therapy due to an affected pump may cause serious adverse health events, including unstable blood pressure, injury (for example: inadequate blood supply or a vital organ injury), and death.
Between January 1, 2021 and June 2, 2023, Getinge/Maquet/Datascope has reported 298 complaints related to this device issue, including two deaths and up to three injuries.
Who May be Affected
- People who receive circulatory support using a Cardiosave Hybrid or Rescue IABP
- Health care personnel providing care that includes the Cardiosave Hybrid or Rescue IABP
What to Do
On July 31, 2023, Datascope/Maquet/Getinge sent all affected customers an Urgent Medical Device Correction letter (described as “Issue 1: Autofill Alarms”).
The letter requested customers to follow these instructions for the Autofill Failure – No Helium and Autofill Failure priority alarms:
- Autofill Failure – No Helium
- Verify that the Cardiosave device has sufficient helium in the system.
- If the Cardiosave is in Hybrid mode (console within a hospital cart), verify the external Helium tank is connected to the cart, is opened, and there is sufficient helium in the tank to support the IABP therapy.
- If the Cardiosave is in Rescue (transport) mode, replenish the IABP’s helium gas supply by placing the IABP console into the hospital cart, or connect the IABP console to a helium refilling station (if available at your facility).
Once these steps are completed, initiate an autofill by pressing the START key, or by pressing the IAB FILL key for 2 seconds.
- Autofill Failure
- Ensure that only one correctly sized IAB extender tubing is tightly connected to the IAB and the IABP, and there are no restrictions in the tubing.
- Check for evidence of blood in the IAB tubing. If any blood is noted or perforation is suspected, the following procedure must be performed immediately:
- Disconnect the catheter extender tubing from the IABP console to allow the balloon to deflate.
- Clamp extracorporeal tubing between white y-fitting and male connector. Place the patient in Trendelenburg as tolerated to guide any residual helium to travel away from the head vessels. Notify physician and prepare for IAB catheter removal. Consider IAB catheter replacement, if the patient’s condition warrants.
- If blood is suspected of having entered the pump, take pump out of service. It should be evaluated before use in another patient by a Getinge or Biomed/Technical Service representative to determine if replacement of contaminated components is necessary.
- If blood is not present, press the START key to refill the IAB and resume pumping.
Getinge/Maquet/Datascope noted that the Autofill Failure – Blood Suspected alarm is caused by a leak/failure in the intra-aortic balloon (IAB) and is outside the scope of this recall.
Contact Information
Customers with questions about this recall should contact their Getinge/Maquet/Datascope representative or call Getinge/Maquet/Datascope Technical Support at 1-888-943-8872, options 4, 2, 1, Monday through Friday, between the hours of 8:00 a.m. and 6:00 p.m. (Eastern Time).
Additional Resources
- Medical Device Recalls database
- Letter to Health Care Provider
- Getinge/Maquet/Datascope - Urgent Medical Device Correction
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.