FY2016 Regulatory Science Report: Locally-Acting Orally-Inhaled and Nasal Drug Products
This section contains only new information from FY2016. For background scientific information and outcomes from previous years on this research topic, please refer to the FY15 Regulatory Science Research Report on Locally-Acting Orally-Inhaled and Nasal Drug Products.
Introduction
Performance of orally inhaled and nasal drug products (OINDPs) is governed by complex interactions between device, formulation, and patient factors. Because existing in vitro methods have limited predictability of these interactions, both development and bioequivalence (BE) demonstration of generic OINDPs is very challenging, time-consuming and expensive, in part due to their reliance on in vivo studies. For this reason, even though there is a current, clear regulatory pathway utilizing the weight-of-evidence approach for BE assessment of OINDPs, the Office of Generic Drugs (OGD) continues to explore new methods to make development and BE assessment of OINDPs more cost- and time-effective in the future. These research initiatives can be broadly separated into four categories:
1) Identification of formulation and device variables which are important for successful development of generic OINDPs;
2) Development of clinically relevant in vitro tools for prediction of in vivo regional drug deposition and dissolution from OINDPs;
3) Development of computational fluid dynamic (CFD) and physiology-based pharmacokinetic (PBPK) models for prediction of the fate of drugs delivered through OINDPs and to assess their applicability in generic OINDP development programs;
4) Identification, validation and standardization of novel techniques that may have the potential to reduce the burden of current BE requirements for generic OINDPs.
Under these initiatives, OGD has explored advanced in vitro/in silico methods such as CFD modeling and clinically relevant in vitro dissolution and deposition tests to understand and predict performance of OIDNPs in a more realistic way. These clinically relevant in vitro methods have shown good in vivo predictability of regional nasal and lung drug deposition and dissolution, as well as local and systemic drug bioavailability of OIDNPs. Some of these methods are described below.
Research
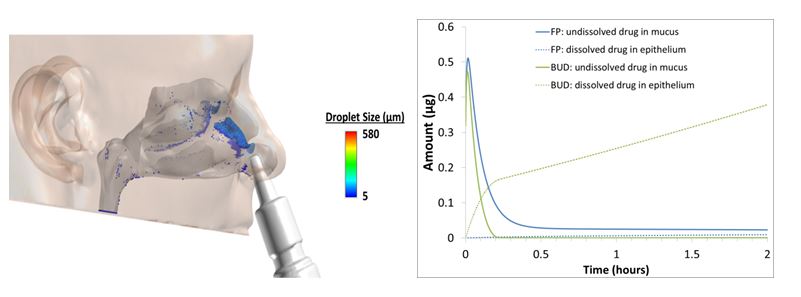
Model simulations will improve our understanding of the absorption of inhaled corticosteroids (ICS) and will support and facilitate development of product-specific recommendations for generic drug development. In support of these objectives, droplet size distributions (DSDs) from three commercial nasal sprays were determined at three different actuation pressures using laser diffraction. Computational fluid dynamics (CFD) models of the nasal passages of healthy and rhinitic subjects were developed to simulate the transport and deposition of droplets from the three nasal spray devices using the DSD information as inputs (Figure 1a). CFD simulation results for the three nasal sprays were used as model inputs to simulate absorption of the corticosteroids fluticasone propionate and budesonide. The PBPK models included the effects of mucociliary clearance, drug dissolution, diffusion through nasal epithelial layers, binding kinetics to the glucocorticoid receptor, liver metabolism, and systemic clearance. The PBPK models can be used to determine which physicochemical properties of the ICS affect the absorption and systemic bioavailability (Figure 1b). In addition, preliminary data from a CFD model of a human nasal cavity also indicated that local delivery of several intranasal corticosteroids (ICSs) may not be sensitive to actuation force when assessed across the range normally seen within a clinical setting.
Figure 1. Left (a): CFD predictions of the droplet deposition pattern of fluticasone propionate nasal spray at a typical actuation pressure in a healthy adult nasal model. Right (b): PBPK model simulations of the dissolution and absorption of fluticasone propionate (FP) and budesonide (BUD) in the nasal mucosa [Figures are courtesy of Applied Research Associates].
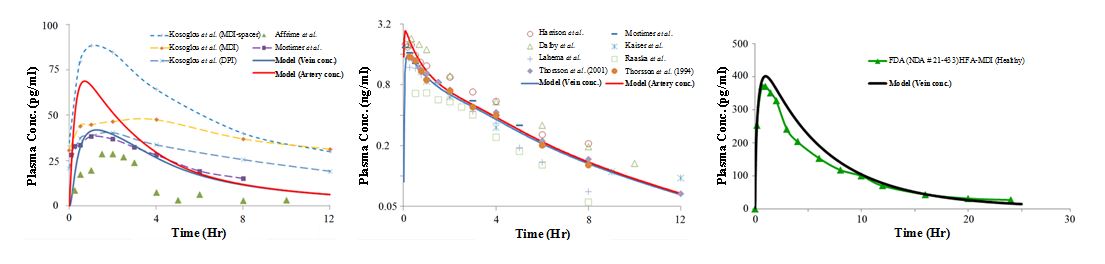
Similarly, work has been done towards the development of lung models which will combine CFD with PBPK modeling (Figure 2). These models will enable the FDA to investigate device and factors that may or may not influence local and systemic delivery (Figure 3) of OIDPs.
Figure 2. Integrated computational framework for pulmonary drug delivery and PBPK-PD simulation [Figures are courtesy of CFD Research Corporation]
Figure 3. The predicted inhaled plasma PK values for momentasone furoate (left), budesonide (center) and fluticasone propionate (right) simulations is shown in comparison with experimental data [Figures are courtesy of CFD Research Corporation].
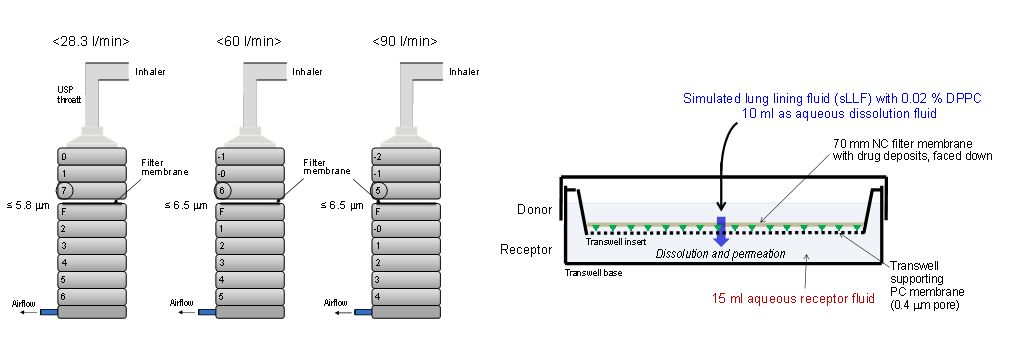
The FDA explored various realistic mouth throat models that are currently used by industry and academia to compare their in vivo predictability when coupled with representative inhalation profiles. The experimental setup and inhalation profiles for a test dry powder inhaler are shown in Figures 4a and 4b. Figure 5 demonstrates that the test dry powder inhaler’s in vitro performance may be dependent on both the mouth-throat geometric size and the inhalation profile. The studies have also evaluated the developed method using a metered dose inhaler and a soft mist inhaler. This method, by incorporating both airway geometry and inhalation profiles, allows assessment of aerodynamic particle size distribution in a clinically relevant manner.
Figure 4. Left (a): Experimental setup to measure total lung dose in vitro and its aerodynamic particle size distributions for drug aerosols delivered from an inhaler. The side inlet of the Nephele Mixing Inlet (NMI) is also connected to a breath simulator and compressed air source. Right (b): Inhalation profiles for the test inhaler representative of subjects trained at fast inhalation condition [Figures are courtesy of Dr. Michael Hindle, Virginia Commonwealth University].
Figure 5. Percent of drug mass depositing on the inhaler, mouth-throat (MT) model, mixing inlet (NMI), and NGI stages when the inhaler was tested with inhalation profiles simulated to represent those used by subjects trained at fast, moderate and slow inhalation conditions [Figures are courtesy of Dr. Michael Hindle, Virginia Commonwealth University].
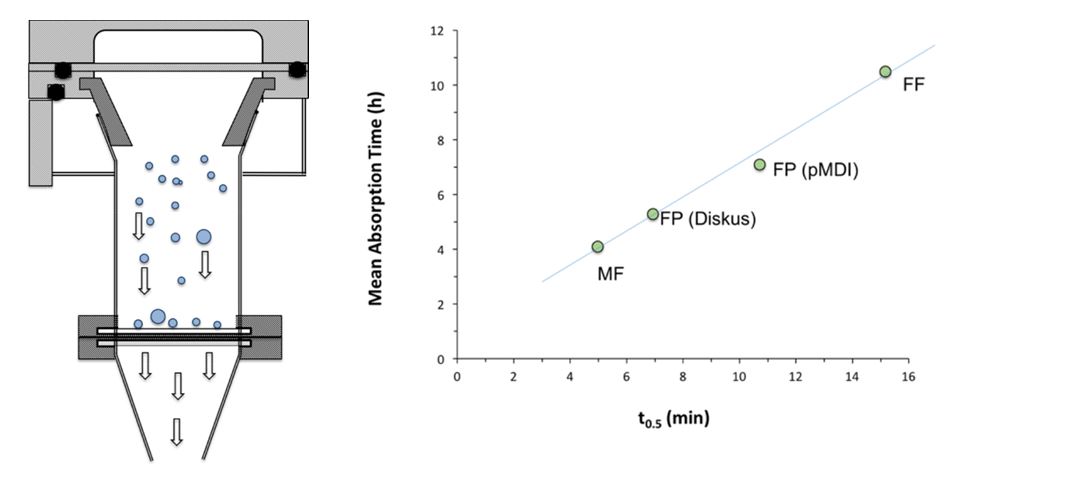
Similarly, OGD has explored three predictive dissolution methods for OIDPs. These methods have been described in the Figures 6 - 9, along with important findings. The preliminary results showed that dissolution tests have good discriminatory capabilities in detecting formulation differences between the different products. In one of the methods shown in Figure 9, a novel apparatus to collect a representative in vitro respirable lung dose for dissolution studies (e.g., ex-mouth-throat dose, impactor stage mass or a dose below a defined impactor stage) is developed. The collection system has been validated for impactor stage mass recovery versus a standard impactor over a range of drug loadings for both dry powder inhaler (DPI) and metered dose inhaler (MDI) products. A good correlation was observed between in vivo mean absorption time (MAT) and the first order half-life of the dissolution profiles of a range of inhaled corticosteroids for the drug collected on the aerosol collection chamber. These research findings indicated that this dissolution method has the potential to be used to investigate both formulation and device related factors and determine the possible influence of dissolution on the absorption behavior of poorly soluble drugs.
Figure 6. Dissolution Method 1 – Left (a): Selected Transwell® system for the evaluation of the dissolution rate of inhalation drugs. Right (b): Differences in the dissolution rate of three model corticosteroids (Bud: budesonide DPI; CIC: ciclesonide MDI; FP: fluticasone propionate DPI) as assessed by the Transwell® system [Figures are courtesy of Dr. Guenther Hochhaus, University of Florida].
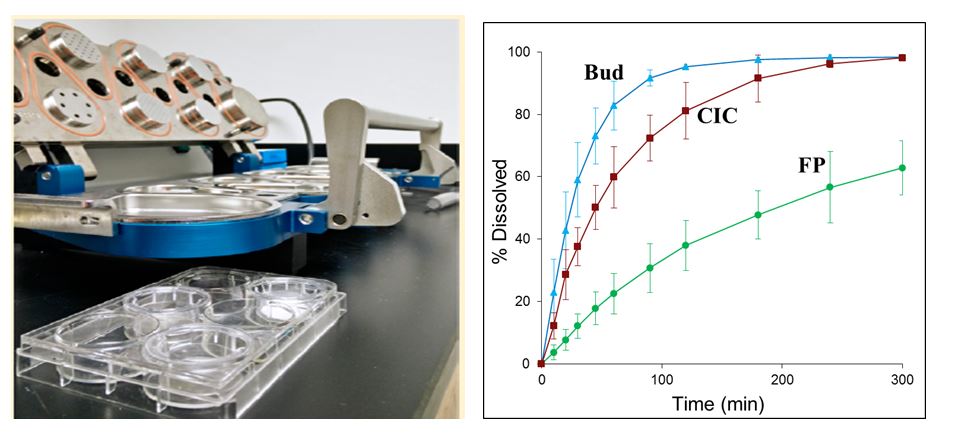
Figure 8. Dissolution Method 2 – Effects of ± 20% differences in A (left) dose-to-lung (DTL), B (center) fraction deposited in the peripheral lung (Fdepp) and C (right) rate constant for dissolution (kdiss) on the plasma concentration-time profiles for fluticasone propionate (FP) following inhalation in healthy subjects, simulated using the kinetic model shown in Figure 3. The solid line profiles are drawn, based on a reference condition, where the model parameters were fixed at DTL = 250 mg, Fdepp = 0.5, and kdiss = 0.2 h-1. The dashed line profiles are the results with ± 20% difference in each of these values, while the remainders were held constant. The Cmax and AUC values are shown as % changes, as a result of these 20% differences [Figures are courtesy of Dr. Mashahiro Sakagami, Virginia Commonwealth University].
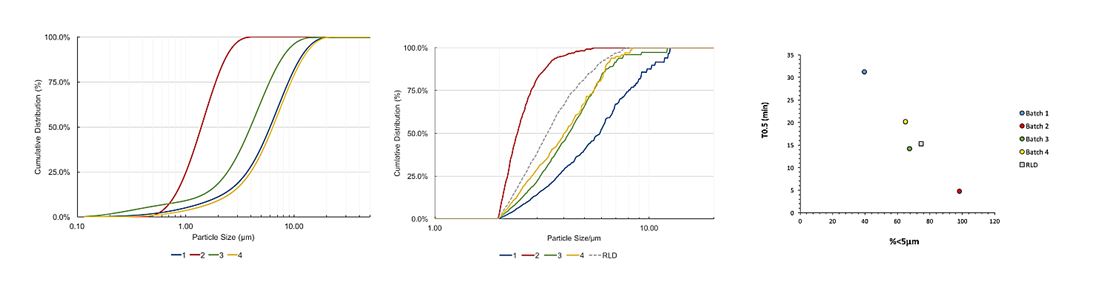
Figure 9. Dissolution Method 3 – Left (a): A schematic of the aerosol collection system. Right (b): Correlation between in vivo mean absorption time (MAT) and the first order half-life of the dissolution profiles of a range of inhaled corticosteroids [Figures are courtesy of Dr. Robert Price, University of Bath] Similarly, OGD is also exploring novel methods such as Morphologically-Directed Raman Spectroscopy (MDRS) for assessment of drug particle size distribution in nasal suspensions; the method has shown good potential during generic product development, especially during formulation optimization. Four batches of mometasone furoate aqueous nasal suspensions were formulated with different drug substance batches of varying particle size, and were analyzed using the MDRS method. As shown in Figure 10b, the MDRS method was able to track the drug substance particle size in the formulation. Also, as shown in Figure 10c, data suggested a good correlation between the percentage by volume less than 5 µm determined by the MDRS method, and in vitro dissolution half-life of the formulated products. Together, these data suggest that the MDRS method in combination with dissolution testing may help generic manufacturers to develop substitutable generic products of aqueous nasal suspensions, and ensure they have control of drug product quality.
Figure 10. Left (a): As-received mometasone furoate drug substance particle size using laser diffraction. Middle (b): The particle size distribution of the formulated drug substance determined by MDRS. Right (c): relationship between the percentage by volume less than 5 µm of the formulated drug substance measured by MDRS and the dissolution half-life of the drug product [Figures are courtesy of Dr. Robert Price, University of Bath].
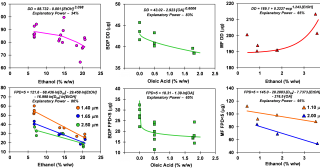
The OGD is also exploring quality by design (QbD) as a means of ensuring the quality and performance of the product through control and increased understanding of material properties and manufacturing processes. For example, the effects of varying ethanol and oleic acid concentrations and the micronized drug particle size D50 were evaluated, based on three commercially available MDI products. Results showed that the delivered dose (DD) was statistically significantly influenced by ethanol concentration for the suspension formulations [albuterol sulfate (AS) and mometasone furoate (MF)], while a solution formulation [beclomethasone dipropionate (BDP)] was significantly influenced by oleic acid concentration. Fine particle dose less than 5 mm (FPD<5) was="" significantly="" dependent="" on="" micronized="" drug="">50 and ethanol concentration for AS formulations. For MF formulations, FPD<5 was="" dependent="" upon="" all="" three="" variables="" evaluated="" (micronized="" drug="">50, ethanol and oleic acid concentrations). For BDP formulations, FPD<5 was="" only="" dependent="" upon="" oleic="" acid="" concentrations.="" figure="" 11="" presents="" the="" impact="" of="" formulation="" parameters="" on="" the="" product="" performance="" for="" each="" model="" system.="" in="" addition,="" statistical="" models="" predicting="" dd="" and=""><5 by="" model="" parameters="" were="" developed,="" explaining="" between="" 34="" and="" 95%="" of="" the="" total="" variation="" seen="" in="" the="" data="" (see="" figure="" 11).="" the="" outcomes="" of="" this="" study="" may="" allow="" for="" better="" definition="" of="" design="" spaces="" for="" dd="" and=""><5, according="" to="" the="" different="" levels="" of="" formulation="" factors.="" the="" systematic="" approach="" utilized="" in="" this="" work="" can="" contribute="" as="" a="" qbd="" tool="" to="" evaluate="" the="" extent="" to="" which="" the="" formulation="" factors="" govern="" the="" aerosolization="" performance="" of="" mdi="" products,="" assisting="" in="" the="" development="" of="" mdi="" formulations="" with="" desired="" product="">
Figure 11: Superimposed data and statistical models for three MDI formulations manufactured according to a design of experiments (DoE) approach for delivered dose (DD) at beginning of canister life and fine particle dose < 5µm=""><5). the="" models="" were="" determined="" based="" on="" the="" formulation="" parameters="" that="" were="" found="" to="" significantly="" impact="" the="" given="" performance="" metric="" [figures="" are="" courtesy="" of="" dr.="" poonam="" aliyah="" sheth,="" recipharm="" laboratories="">
OGD’s research initiatives have attracted wide interest from the industry; our grantees and contractors as well as FDA representatives have been invited to give presentations on these new methods at conferences attended by industry, academia, and regulators. Therefore, outcomes of these research initiatives have been made available continuously to public through presentations and publications.
ORS staff facilitating research in this area
- Renishkumar Delvadia, Denise Conti, Oguntimein Oluwamurewa (Murewa), Kimberly Witzmann, Andrew Babiskin, Ross Walenga
Projects and Collaborators
- Development of Clinically Relevant in Vitro Performance Test for Generic OIDPs
- Site PI: Michael Hindle (Virginia Commonwealth University)
- Grant #: 1U01FD005231-01
- Pharmacokinetic Comparison of Locally Acting Orally Inhaled Drug Products
- Study PI: Juergen Bulitta (University of Florida)
- Contract #: HHSF223201610099C
- Comprehensive Evaluation of Formulation Effects on Metered Dose Inhaler Performance
- Site PI: Guenther Hochhaus (University of Florida)
- Grant #: 1U01FD004943-01, 5U01FD004943-05
- An optimized dissolution test system for orally inhaled drugs: development and Validation
- Site PI: Guenther Hochhaus (University of Florida)
- Grant #: 1U01FD004950-01
- In vitro fluid capacity-limited dissolution testing and its kinetic relation to in vivo clinical pharmacokinetics for orally inhaled drug products
- Site PI: Masahiro Sakagami (Virginia Commonwealth University)
- Grant #: 1U01FD004941-01
- Development of in vivo predictive dissolution technique to understand the clinical based
- Site PI: Robert Price (University of Bath)
- Grant #: 1U01FD004953-01
- Study to investigate the sensitivity of pharmacokinetics in detecting differences in physicochemical properties of the active in suspension nasal products for local action
- Study PI: Guenther Hochhaus (University of Florida)
- Contract #: HHSF223201310220C
- Pharmacokinetic research study on the effects of different protective packaging on the stability of fluticasone propionate capsules for inhalation
- Study PI: Guenther Hochhaus (University of Florida)
- Contract #: HHSF223201300479A
- Evaluation of formulation and device changes on in vitro performance of dry powder inhaler (DPI)
- Study PI: Robert Price (University of Bath)
- Contract #: HHSF223200910017C
- Quality by design of orally inhaled drug products: chemistry, manufacturing and controls
- Study PI: Robert Price (University of Bath)
- Grant #: 1U01FD004321-01
- A predictive multiscale computational tool for simulation of lung absorption and pharmacokinetics and optimization of pulmonary drug delivery
- Site PI: Narender Singh
- Grant #: 1U01FD005214-01
- Development of hybrid CFD-PBPK models for absorption of intranasal corticosteroids
- Site PI: Jeff Schroeter
- Grant #: 1U01FD005201-01
Presentations
- Kimbell JS, Stricklin D, and Schroeter JD. Simulating nasal spray deposition: Effects of spray nozzle presence in the nasal vestibule. International Society for Aerosols in Medicine Conference, Munich, Germany, May 30 – June 3, 2016 (poster presentation).
- Schroeter JD. Prediction II: Computational Fluid Dynamics, as part of a workshop titled Bridging the Gap from Science to Clinical Efficacy: Imaging, Modelling, and Physiology of Aerosols and the Lung. International Society for Aerosols in Medicine Conference, Munich, Germany, May 30 – June 3, 2016 (platform presentation).
- Schroeter JD, Stricklin D, Kimbell JS, Delvadia RR, and Zhang X. A physiologically-based pharmacokinetic model framework to estimate systemic bioavailability of fluticasone propionate nasal spray. Respiratory Drug Delivery 2016, Scottsdale, AZ, April 17-21, 2016 (poster presentation).
- Schroeter JD, Kimbell JS, Saluja B, Delvadia RR, Vallorz III EL, and Sheth P. The impact of actuation force on droplet size distribution and spray duration of three commercially available nasal sprays. Respiratory Drug Delivery 2016, Scottsdale, AZ, April 17-21, 2016 (poster presentation).
- Kimbell JS, Schroeter JD, Sheth P, Tian G, Delvadia RR, Saluja B, and Walenga R. Effect of actuation force on simulated regional nasal spray deposition in a healthy nasal cavity. Respiratory Drug Delivery 2016, Scottsdale, AZ, April 17-21, 2016 (poster presentation).
- Wei X and Byron PR: Clinically relevant in vitro performance tests for powder inhalers. RDD Asia 2016, Goa, India, November 2016 (Podium presentation).
- Byron PR, Wei X, Bormann K: Estimating aerosol size distributions and doses entering the trachea. Respiratory Drug Delivery 2016, Scottsdale, Arizona, April 2016 (Podium presentation).
- Huynh B, Wei X, Byron PR: Evaluating electrostatic drug deposition in plastic mouth-throat models with Budelin® Novolizer®”. Respiratory Drug Delivery 2016, Scottsdale, Arizona, April 2016 (Poster presentation).
- Wei X, Bormann K, Byron PR: Predicting variations in aerodynamic particle size distribution of the lung dose from Budelin Novolizer”. RDD Europe 2015, Antibes, France, May 2015 (Poster presentation).
- Azimi M, Longest PW, Shur J, Price R and Hindle M: Clinically relevant in vitro tests for the assessment of innovator and generic nasal spray products, Drug Delivery to the Lungs, Scotland, UK, December 2016 (Podium presentation).
- Azimi M, Hindle M and Longest PW: In vitro deposition of a nasal spray product using two realistic airway models, American Association of Pharmaceutical Scientists, Denver, CO, USA, November 2016 (Poster presentation).
- Longest PW, Rygg A, Hindle M: Bioequivalence Testing: Can Systemic Pharmacokinetic Profiles from Corticosteroid Nasal Sprays Be Used to Elucidate Local Drug Deposition within the Nose? Respiratory Drug Delivery 2016, Scottsdale, Arizona, April 2016 (Podium presentation).
- Azimi M, Hindle M, Longest PW and Walenga RL: Comparison of the in vitro deposition of Nasonex(R) nasal spray product in two realistic nasal airway models. Respiratory Drug Delivery 2016, Scottsdale, Arizona, April 2016 (Poster presentation).
- Azimi M, Longest P, Hindle M: Towards clinically relevant in vitro testing of locally acting nasal spray suspension products, RDD Europe 2015, Nice, France, May 2015 (Podium presentation).
- Kannan et al., A Multiscale framework for Computational Inhalation Pharmacology., 2nd International Conference on Respiratory & Pulmonary Medicine, Chicago, IL, 17-18 Oct. 2016 (Podium presentation and poster).
- Conti DS, Holt J, Sheth P, Sandell D, Hickey A, Saluja B. The Effects of Formulation Factors on the Aerosolization Performance of Metered Dose Inhalers. FDA Generic Drug Science Day, Silver Spring, MD (2016) [meeting abstract and poster presentation accepted].
- Conti DS, Holt J, Sheth P, Sandell D, Hickey A, Saluja B. The Effects of Formulation Factors on the Aerosolization Performance of Metered Dose Inhalers. American Institute of Chemical Engineers (AIChe) Annual Meeting, San Francisco, CA (2016) [meeting abstract and poster presentation accepted]
- Sheth P, Sandell D, Svensson M, Vallorz III EL, Sullivan JB, Saluja B, Conti DS. The Influence of Formulation Variables on Mometasone Furoate pMDIs. Respiratory Drug Delivery (RDD) Conference, Phoenix, AZ, Volume 2: 285-290 (2016) [meeting abstract and poster presentation].
- Sheth P, Vallorz III EL, Susick R, Menzeleev R. A Dual-Internal Standard LC-UV-MSD Method to Improve Accuracy and Efficiency of Characterizing Mometasone Furoate MDIs. American Association of Pharmaceutical Scientists (AAPS) Annual Meeting, Orlando, FL (2015) [meeting abstract and poster presentation].
- Sandell, D. Varying Particle Size and Excipient Levels for Three MDIs: Effects on In-Vitro Performance. 4th Medicon Valley Inhalation Symposium (MVIC), Medicon Village, Lund, Sweden (2015) [oral presentation].
- Conti DS, Holt J, Sandell D, Schroeter J, Hickey A, Lee S, Saluja B. Systematic Evaluation of Formulation Factors on Aerosolization Performance of Metered Dose Inhalers. FDA Science Forum, Silver Spring, MD (2015) [meeting abstract and poster presentation].
- Holt J, Sandell D, Hickey A, Vallorz E, Straughn K. The Influence of Formulation Variables on the Performance of a Beclomethasone Dipropionate Metered Dose Inhaler. Respiratory Drug Delivery (RDD) Europe Conference, Palais des Congres d'Antibes, Nice, France, Volume 2: 275-280 (2015) [meeting abstract and poster presentation].
Publications
- Schroeter JD, Stricklin D, Kimbell JS, Delvadia RR, and Zhang X. (2016). A physiologically-based pharmacokinetic model framework to estimate systemic bioavailability of fluticasone propionate nasal spray. Respiratory Drug Delivery 2016 Proceedings.
- Schroeter JD, Kimbell JS, Saluja B, Delvadia RR, Vallorz III EL, and Sheth P. (2016). The impact of actuation force on droplet size distribution and spray duration of three commercially available nasal sprays. Respiratory Drug Delivery 2016 Proceedings.
- Kimbell JS, Schroeter JD, Sheth P, Tian G, Delvadia RR, Saluja B, and Walenga R. (2016). Effect of actuation force on simulated regional nasal spray deposition in a healthy nasal cavity. Respiratory Drug Delivery 2016 Proceedings.
- Wei X, Hindle M, Delvadia RR, Byron PR: 2016. In vitro tests for aerosol deposition. V: Using realistic testing to estimate variations in aerosol properties at the trachea. Journal of Aerosol Medicine and Pulmonary Drug Delivery (submitted).
- Wei X and Byron PR: Clinically relevant in vitro performance tests for powder inhalers. RDD Asia 2016. (In Press).
- Delvadia RR, Wei X, Longest PW, Venitz J, Byron PR: 2016. In vitro tests for aerosol deposition. IV: Simulating variations in human breath profiles for realistic DPI testing. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 29 (2):196-206.
- Byron PR, Wei X, Bormann K: Estimating aerosol size distributions and doses entering the trachea. Respiratory Drug Delivery 2016. 1: 109-116.
- Huynh B, Wei X, Byron PR: Evaluating electrostatic drug deposition in plastic mouth-throat models with Budelin® Novolizer®. Respiratory Drug Delivery 2016. 3: 631-636.
- Wei X, Bormann K, Byron PR: Predicting variations in aerodynamic particle size distribution of the lung dose from Budelin® Novolizer®. RDD Europe 2015. 2: 533-536.
- Rygg A, Hindle M, Longest PW: 2016. Linking suspension nasal spray drug deposition patterns to pharmacokinetic profiles: a proof of concept study using computational fluid dynamics. Journal of Pharmaceutical Sciences. 105 (6): 1995-2004
- Rygg A, Hindle M, Longest PW: 2015. Absorption and clearance of pharmaceutical aerosols in the human nose: Effects of nasal spray suspension particle size and properties. Pharmaceutical Research. 33(4):909-921.
- Rygg A. and Longest PW: 2015. Absorption and clearance of pharmaceutical aerosols in the human nose: Development of a CFD model. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 29 (5): 416-431.
- Azimi M, Longest P, Hindle M: Towards clinically relevant in vitro testing of locally acting nasal spray suspension products, RDD Europe 2015. 1: 121-130.
- Longest PW, Rygg A, Hindle M: Bioequivalence Testing: Can Systemic Pharmacokinetic Profiles from Corticosteroid Nasal Sprays Be Used to Elucidate Local Drug Deposition within the Nose? Respiratory Drug Delivery 2016. 1: 175-184.
- Azimi M, Hindle M, Longest PW and Walenga RL: Comparison of the in vitro deposition of Nasonex(R) nasal spray product in two realistic nasal airway models. Respiratory Drug Delivery 2016. 3: 617-622.
- Azimi M, Longest, PW, Shur J, Price R and Hindle M. (2016) Clinically relevant in vitro tests for the assessment of innovator and generic nasal spray products, Journal of Aerosol Medicine and Pulmonary Drug Delivery (In Press).
- Kannan et al., A Quasi-3D wire approach to model pulmonary airflow in human airways., International Journal for Numerical Methods in Biomedical Engineering, 2016. DOI: 10.1002/cnm.2838
Outcomes
- Product-specific guidance on Budesonide; Formoterol fumarate dihydrate inhalation aerosol metered (Jun 2015)
- Product-specific guidance on Aclidinium bromide inhalation powder (Sept 2015)
- Product-specific guidance on Fluticasone propionate nasal spray metered (Sep 2015)
- Product-specific guidance on Formoterol fumarate inhalation powder (Sept 2015)
- Product-specific guidance on Mometasone furoate nasal spray metered (Sep 2015)
- Product-specific guidance on Beclomethasone dipropionate inhalation aerosol metered (Jan 2016)
- Product-specific guidance on Ciclesonide inhalation aerosol metered (Jan 2016)
- Product-specific guidance on Formoterol fumarate; Mometasone furoate inhalation aerosol metered (Jan 2016)
- Product-specific guidance on Fluticasone furoate inhalation powder (Apr 2016)
- Product-specific guidance on Fluticasone furoate; Vilanterol trifenatate inhalation powder (Apr 2016)
- Product-specific guidance on Indacaterol maleate inhalation powder (Apr 2016)
- Product-specific guidance on Mometasone furoate inhalation aerosol metered. (Apr 2016)
- Revision of Product-specific guidance on Azelastine hydrochloride and fluticasone propionate nasal spray metered (Jun 2016)
- Product-specific guidance on Olopatadine hydrochloride nasal spray metered (Oct 2016)
- Product-specific guidance on Triamcinolone acetonide nasal spray metered (Oct 2016)
- Product-specific guidance on Umeclidinium bromide inhalation powder (Oct 2016)