Human Drug Common Entry Errors
Introduction
The Center for Evaluation and Research (CDER) is responsible for overseeing the drug program including over-the-counter, prescription drugs, biological therapeutics, and generic drugs.
The law defines a drug, in part, as: “intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease” and “articles (other than food) intended to affect the structure or any function of the body of man or other animals. “This definition also includes components of drugs, such as active pharmaceutical ingredients.
To expedite entry screening of medical drug products by the Predictive Risk-based Evaluation for Dynamic Import Compliance Targeting (PREDICT) system, importers and entry filers should provide accurate product codes, all relevant affirmations of compliance (AofC) and accurate identifiers for firms, in addition to the general entry data.
When applicable entry data is supplied electronically, completely, and accurately, it can be used by the PREDICT system to “look-up” the information in the FDA’s databases, validate the information, and issue a system “May Proceed” if the line is not held due to other screening criteria.
When this additional entry information is not provided, or if it is provided incorrectly, the automated look-up will fail, and the entry line may be subject to delays in processing.
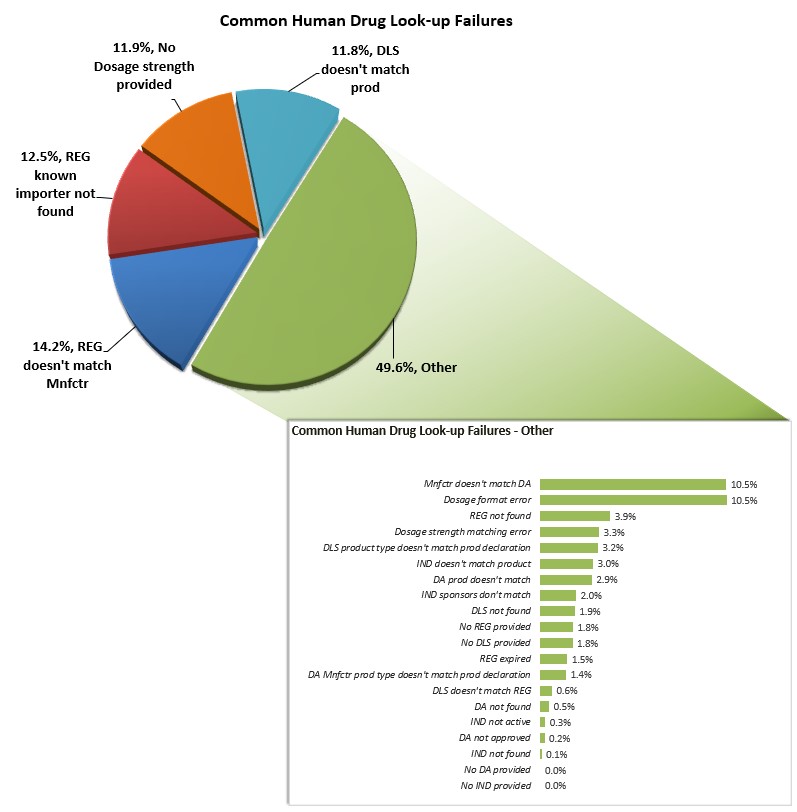
The following chart shows the most common look-up errors for medical drug product entries from April 1, 2025 to September 30, 2025, and provides explanations on how to address errors.
When the entry data provided is not complete and/or accurate, the entry line may be subject to delays in processing. The table below provides additional information regarding these common errors to help you provide accurate and complete information to the FDA.
Common Human Drug Look-up Errors that Result in a Failed Look-up
| Error | Description | Additional Information |
|---|---|---|
| DA Mnfctr prod type does not match prod declaration | The product type/manufacturer combination declared on the entry is not approved under the drug application number (DA) provided. For example, the manufacturer is approved under the DA as a finished dosage manufacturer however the product type declared is a bulk ingredient.). Verify the product, manufacturer and DA declared are correct. | Drug applications contain information on the manufacturing responsibilities performed by all facilities associated with the application. This includes manufacturing functions specific to both drug substances and drug product manufacturers. For more information on drug requirements verified at the time of importation, see Import Basics - Human Drugs To confirm the DA matches the product and manufacturer contact the responsible party. |
| DA not found | The drug application number (DA) transmitted could not be found in FDA's database. Verify the DA provided is accurate and in the correct format. | For help on DA definition and format, see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide. The FDA cannot confirm, verify, or provide DAs to individuals that are not the applicant. To confirm the DA is accurate contact the responsible party. |
| DA not approved | The drug application number (DA) provided is not approved. Verify the DA is accurate. | For more information on drug requirements verified at the time of importation, see Import Basics - Human Drugs. To confirm the DA is accurate contact the responsible party. |
| DA prod does not match | The product covered under the drug application number (DA) provided does not match the product declared on the entry. Verify the correct DA was provided and the product declared is accurate. | For more information on drug requirements verified at the time of importation, see Import Basics - Human Drugs. To confirm the DA is accurate contact the responsible party. |
| DLS product type does not match prod declaration | The product type (e.g. OTC, bulk ingredient, prescription) for the drug listing number (DLS) provided does not match the declared product type (declared using the PIC code or 5th character of the FDA product code) on the entry. Verify that the correct DLS was provided and that the product code submitted is accurate. |
|
| DLS does not match prod | The product proprietary or non-proprietary name covered by the drug listing number (DLS) provided does not match the product declared on the entry. Verify the correct DLS was provided, and the product declared is accurate. | To find publicly available drug listing information for certain active and certified finished and unfinished drugs, including proprietary and non-proprietary names, search the National Drug Code Directory The FDA cannot confirm, verify, or provide DLS information to individuals other than the listing holder. To confirm the DLS contact the official correspondent of the firm that is required to list. For more information on drug requirements verified at the time of import, See Import Basics - Human Drugs. |
| DLS does not match REG | The drug listing number (DLS) provided does not match with the drug firm registration number (REG) provided. Verify that the correct DLS and REG have been provided. | For more information on drug requirements verified at the time of importation, see the Import Basics - Human Drugs page. To find a REG for public DUNS and FEI numbers, search the Drug Establishments Current Registration. To find a publicly available drug listing information for certain active and certified finished and unfinished drugs, search the National Drug Code Directory. Contact the official correspondent of the firm required to list and register to verify the DLS and REG. |
| DLS not found | The drug listing number (DLS) transmitted could not be found in FDA's database. Verify the DLS provided is accurate and in the correct format. | For help on DLS definition and format, see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide. To find a publicly available DLS for certain active and certified finished and unfinished drugs, search National Drug Code Directory. The FDA cannot confirm, verify, or provide DLS information to individuals other than the listing holder. To confirm the DLS contact the official correspondent of the firm that is required to list. |
| Dosage format error | The dosage strength declared on the entry does not match the declared dosage form. (e.g. the product is declared as a finished dosage form tablet however the dosage strength is listed in __mg/ml__) Verify the correct dosage strength and dosage form are being submitted and that they match with the product being declared. | To find publicly available drug listing information for certain active and certified finished and unfinished drugs, including the strength, search the National Drug Code Directory. The FDA cannot confirm, verify, or provide DLS information to individuals other than the listing holder. To verify the DLS information contact the official correspondent of the firm that is required to list. For more information see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide and Import Basics - Human Drugs. |
| Dosage strength matching error | The dosage strength declared on the entry does not match with the dosage strength for the drug listing number (DLS) provided. Verify the correct dosage strength is being provided, that it matches the format/units in the drug listing, and that the DLS provided is correct. | To find publicly available drug listing information for certain active and certified finished and unfinished drugs, including the strength, search the National Drug Code Directory. The FDA cannot confirm, verify, or provide DLS information to individuals other than the listing holder. To verify the DLS information contact the official correspondent of the firm that is required to list. For finished dosage form drug products transmit the percent of active ingredient in the product. For active pharmaceutical ingredients (API)/ bulk drug substances, transmit the percent identification of the API. See the FDA ACE Supplemental Guide, section 7.4 Record Identifier PG04, for additional information. |
| IND does not match product | The product name or drug covered by the Investigational New Drug application number (IND) provided does not match the product declared on the entry. Verify the correct IND was provided and that the product declared is accurate. | The FDA cannot confirm, verify, or provide IND information to individuals other than the sponsor. To confirm the IND contact the responsible party. |
| IND not active | The Investigational New Drug application number (IND) provided is not active (in effect) in FDA's database. Verify that the IND being declared is the most current IND and is accurate. | For more information on drug requirements verified at the time of importation see Import Basics - Human Drugs. The FDA cannot confirm, verify, or provide IND information to individuals other than the sponsor. To confirm the IND, contact the responsible party. |
| IND not found | The Investigational New Drug application number (IND) transmitted could not be found in FDA's database. Verify the IND number provided is accurate and in the correct format. | For help on IND definition and format, see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide. The FDA cannot confirm, verify, or provide IND information to individuals other than the sponsor. To confirm the IND, contact the responsible party. |
| IND sponsors don't match | The importer or consignee declared on the entry does not match the sponsor, qualified investigator, or the domestic agent of the foreign sponsor for the Investigational New Drug application number (IND) provided. Verify the correct IND was provided and the consignee and/or importer are accurate | Contact the responsible party to confirm the IND and firm information. |
| Mnfctr does not match DA | The manufacturer declared on the entry does not match an approved manufacturer for the drug application number (DA) submitted. Verify the manufacturer being declared is accurate and that the DA submitted is accurate. | For more information on drug requirements verified at the time of importation, see Import Basics - Human Drugs. To confirm the DA is accurate contact the responsible party. |
| No DA provided | Based on the product declaration a drug application number (DA) is required; however, no DA was provided for the entry. Verify the product and product code declared are accurate, and the correct intended use code (IUC) was submitted. Provide the DA for the product offered for import. | For product code assistance, see the Product Codes and Product Code Builder page. For help on intended use codes, DA definition, and DA format, see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide. To determine the appropriate DA contact the responsible party. |
| No Dosage strength provided | No dosage strength was provided for this entry, or the dosage strength was provided in a way that FDA's system cannot determine what was being declared. Provide accurate dosage strength on entries for the product being declared. | For information on transmitting dosage strength, see the FDA ACE Supplemental Guide, section 7.4 Record Identifier PG04. For finished dosage form drug products transmit the percent of active ingredient in the product. For active pharmaceutical ingredients (API)/ bulk drug substances, transmit the percent identification of the API |
| No DLS provided | Based on the product declaration a drug listing number (DLS) or National Drug Code (NDC) should have been submitted using the DLS Affirmation of Compliance code; however, no DLS was provided for the entry. Verify the product and product code declared is accurate, and the correct intended use code (IUC) was submitted. Provide the DLS for the product offered for import. | For product code assistance, see the Product Codes and Product Code Builder page. For help on intended use codes, DLS definition, and DLS format, see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide. To determine the DLS contact the responsible party. For more information on drug listing requirements see Electronic Drug Registration and Listing System and Import Basics - Human Drugs. |
| No IND provided | Based on the product declaration an Investigational New Drug application number (IND) should have been submitted; however, no IND was provided for the entry. Verify that the product and product code declared is accurate, and the correct intended use code (IUC) was submitted. Provide the IND for the product offered for import. | For product code assistance, see the Product Codes and Product Code Builder page. For help on intended use codes, IND definition, and IND format, see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide. To determine the IND, contact the responsible party. |
| No REG provided | Based on the product declaration a drug firm registration number (REG) should have been submitted; however, no REG was provided for the entry. Verify the product and product code declared is accurate, and the correct intended use code (IUC) was submitted. Provide the REG. | For product code assistance, see the Product Codes and Product Code Builder page. For help on intended use codes, REG definition, and REG format, see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide. To determine the REG, contact the responsible party. For more information on drug registration requirements see Electronic Drug Registration and Listing System and Import Basics- Human Drugs. |
| REG does not match Mnfctr | The drug firm registration number (REG) provided does not match the manufacturer declared on the entry. Verify that the REG and manufacturer being declared are accurate. | See the Drug Establishments Current Registration site for public DUNS and FEI numbers and information. To confirm the REG, contact the responsible party. |
| REG expired | The drug firm registration number (REG) provided is not current. Verify that the REG provided is the most current and accurate REG. | For more information on drug firm registration requirements see Electronic Drug Registration and Listing System and Import Basics- Human Drugs. See the Drug Establishments Current Registration site for public DUNS and FEI numbers and information. To confirm the REG, contact the responsible party. |
| REG known importer not found | The consignee or importer is not included as a known importer in the registration for the drug firm registration number (REG) provided.
| For more information on drug firm registration requirements see Electronic Drug Registration and Listing System and Import Basics- Human Drugs. The FDA cannot confirm, verify, or provide REG information to individuals other than the registered firm and their U.S. Agent. Contact the official correspondent of the foreign manufacturer for information on the importers and consignees identified in their drug registration. |
| REG not found | The submitted drug firm registration number (REG) could not be found in FDA's database. Verify the REG provided is accurate and in the correct format. | For help on REG definition and format, see the Industry Quick Reference Guide to the FDA ACE Supplemental Guide. See the Drug Establishments Current Registration site for public DUNS and FEI numbers and information. The FDA cannot confirm, verify, or provide REG information to individuals other than the registered firm and their U.S. Agent. To confirm the REG, contact the official correspondent of the firm that is required to register. |