Outbreak Investigation of Listeria monocytogenes: Enoki Mushrooms (November 2022)
FDA’s investigation is complete. CDC declares outbreak over.
Download in Simplified Chinese
The US Food and Drug Administration (FDA), along with Centers for Disease Control and Prevention (CDC) as well as state and local partners, investigated an outbreak of Listeria monocytogenes infections linked to enoki mushrooms. FDA identified enoki mushrooms distributed by Utopia Foods, Inc. of Glendale, New York, and imported from China, and enoki mushrooms labeled as "Producer: Shandong Youhe Biotechnology, Co.," with an address in China and "Distributed By: Sun Hong Foods, Inc." as likely sources of illnesses in this outbreak. Enoki mushrooms are long thin white mushrooms, usually sold in clusters. They are especially popular in East Asian cuisine and are also known as enokitake, golden needle mushrooms, futu, seafood mushrooms, or lily mushrooms.
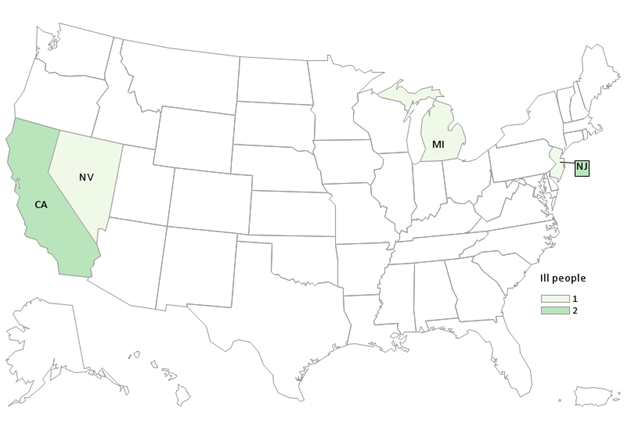
As of April 7, 2023, CDC reports this outbreak is over. Five people in four states (CA, MI, NJ, NV) were sickened with five people reporting hospitalization. There was one pregnancy-associated illness and no reported deaths.
During this investigation, FDA leveraged ongoing surveillance sampling efforts. Several import and retail samples were collected and tested by FDA and/or state and local partners. Laboratory results indicated that many enoki products sampled were contaminated with Listeria. On January 17, 2023, FDA reported a positive import sample of enoki mushrooms that matched both outbreak strains and resulted in a voluntary recall expansion from Utopia Foods, Inc.
Additional sample collection and analysis conducted by the Maryland Department of Health identified both outbreak strains of Listeria in two product samples of enoki mushrooms. The products that tested positive were sold in a 7.05-oz (200g) clear plastic package with a brown and green label and included a label on the back of the package that states: “Producer: Shandong Youhe Biotechnology Co.,” with an address in China, and “Distributed By: Sun Hong Foods, Inc.”
While the outbreak has ended, FDA continues to assess the risk of Listeria contamination in enoki mushrooms.
Further, FDA has added enoki mushrooms from China to a country wide import alert (Import Alert (IA) #25-21). As stated in the Import Alert, FDA Import Divisions may subject shipments of enoki mushrooms from Republic of Korea and China to Detention Without Physical Examination (DWPE). FDA import alerts inform the FDA's field staff and the public that the agency has enough evidence to allow for DWPE of products that appear to be in violation of the FDA's laws and regulations. DWPE helps to prevent potentially violative products from being distributed in the United States.
In addition, after the 2020 outbreak linked to enoki mushrooms, FDA began implementing a Strategy to Help Prevent Listeriosis and Salmonellosis Outbreaks Associated with Imported Enoki and Imported Wood Ear Mushrooms, to protect public health. This prevention strategy is an affirmative, deliberate approach undertaken by FDA and stakeholders to help limit or prevent future outbreaks linked to certain FDA-regulated foods.
Recommendation
Although this outbreak investigation has ended, FDA and CDC are working to better understand the risk of Listeria monocytogenes contamination in enoki mushrooms.
CDC and FDA advise people who are pregnant, aged 65 or older, or have a weakened immune system to cook enoki mushrooms thoroughly, and to:
- Avoid eating enoki mushrooms raw.
- Keep raw enoki mushrooms separate from foods that won’t be cooked.
- Wash your hands, items, and surfaces that have touched raw enoki mushrooms.
Restaurants should cook enoki mushrooms thoroughly before serving to customers, follow FDA’s safe handling and cleaning advice, and use extra vigilance in cleaning and sanitizing any surfaces and containers that may have come in contact with these products.
This advice is based on the following information:
- Two recent multistate Listeria outbreaks have been linked to enoki mushrooms: This outbreak, and the first known Listeria outbreak in the United States linked to enoki mushrooms in 2020.
- During this investigation, FDA leveraged ongoing surveillance sampling efforts. Several import and retail samples were collected and tested by FDA and/or state and local partners and many were contaminated with Listeria. Some samples contained high levels of the bacteria.
- More than 20 recalls of enoki mushrooms due to potential Listeria contamination have been conducted since 2020.
Case Count Map Provided by CDC
Case Counts
Total Illnesses: 5
Hospitalizations: 5
Deaths: 0
Last illness onset: February 3, 2023
States with Cases: CA (2), MI (1), NJ (1), NV (1)
Product Distribution: Nationwide
Useful Links
- Utopia Foods, Inc. Recall
- Utopia Foods, Inc. Expanded Recall
- CDC Investigation Notice
- FDA Safety Alert For Sung Hong Enoki Mushrooms
- Maryland Department of Health Advisory
- What is Listeria?
- Food Safety Tips for Consumers & Retailers During an Outbreak
- FDA Expands Country-Wide Import Alert for Enoki Mushrooms to China
- FDA’s Strategy to Help Prevent Listeriosis and Salmonellosis Outbreaks Associated with Imported Enoki and Imported Wood Ear Mushrooms
- Who to Contact
Previous Updates
February 9, 2023
FDA, along with CDC and state and local partners, is investigating an outbreak of Listeria monocytogenes infections linked to enoki mushrooms. FDA has identified enoki mushrooms distributed by Utopia Foods, Inc. of Glendale, New York and imported from China, and enoki mushrooms labeled as "Producer: Shandong Youhe Biotechnology, Co.," with an address in China and "Distributed By: Sun Hong Foods, Inc." as likely sources of illnesses in this outbreak. Enoki mushrooms are long thin white mushrooms, usually sold in clusters. They are especially popular in East Asian cuisine and are also known as enokitake, golden needle, futu, seafood, or lily mushrooms.
As of January 18, 2023, CDC reports three illnesses included in this outbreak. Through ongoing import and product sampling of enoki mushrooms, two strains of Listeria monocytogenes detected on enoki mushroom products have been determined through Whole Genome Sequencing (WGS) to be the same strains of Listeria monocytogenes linked to illnesses in this outbreak. Both strains are included in this outbreak investigation.
On January 17, 2023, FDA reported a positive import sample of enoki mushrooms that matched both outbreak strains and resulted in a voluntary recall expansion from Utopia Foods, Inc. Additional sample collection and analysis conducted by the Maryland Department of Health have also identified both outbreak strains of Listeria monocytogenes in two product samples of enoki mushrooms. These products that tested positive are sold in a 7.05-oz (200g) clear plastic package with a brown and green label and include a label on the back of the package that states: “Producer: Shandong Youhe Biotechnology Co.,” with an address in China, and “Distributed By: Sun Hong Foods, Inc.”
On December 17, 2022, FDA issued a safety alert for enoki mushrooms labeled as "Producer: Shandong Youhe Biotechnology, Co.," with an address in China and "Distributed By: Sun Hong Foods, Inc.," as a result of a positive product sample collected by Missouri state partners. At that time, these products were not linked to an active outbreak. The strain of Listeria found in these products matches one of the two strains linked to illnesses in this outbreak. FDA’s safety alert has been updated with the most recent information linking these products to an ongoing outbreak investigation.
FDA’s investigation is ongoing to determine a potential source of contamination and whether any other products are contaminated or linked to illnesses. Additional information will be provided as it becomes available.
Products Labeled as Producer: Shandong Youhe Biotechnology, Co.," with an address in China and "Distributed By: Sun Hong Foods, Inc."
These products that tested positive include a label on the back of the package that states:
- "Producer: Shandong Youhe Biotechnology"
- "Distributed By: Sun Hong Foods, Inc."
FDA is advising consumers, restaurants, and retailers not to eat, sell, or serve enoki mushrooms labeled as "Producer: Shandong Youhe Biotechnology, Co.," with an address in China and "Distributed By: Sun Hong Foods, Inc."
January 18, 2023
FDA, along with CDC and state and local partners, is investigating an outbreak of Listeria monocytogenes infections linked to enoki mushrooms. FDA has identified imported enoki mushrooms distributed by Utopia Foods, Inc. of Glendale, New York, as a likely source of illnesses in this outbreak. Enoki mushrooms are long thin white mushrooms, usually sold in clusters. They are especially popular in East Asian cuisine and are also known as enokitake, golden needle, futu, seafood, or lily mushrooms.
As of January 18, 2023, CDC reports three illnesses included in this outbreak. Two sick people reported eating enoki mushrooms or eating at restaurants with menu items containing enoki mushrooms. One patient did not report eating enoki mushrooms but reported shopping at various Asian grocery stores.
Through ongoing sampling efforts, FDA and state partners have been collecting and testing samples of enoki mushrooms. An import sample of enoki mushrooms branded as Utopia Foods, Inc. was collected by FDA and was reported as being positive for Listeria monocytogenes. Whole Genome Sequencing (WGS) analysis determined that the strain of Listeria found in this sample matches the strain of Listeria linked to two illnesses in this outbreak. FDA also detected an additional strain of Listeria monocytogenes in this sample, which is linked to one additional illness. Both strains are now included in this outbreak investigation.
As a result of this testing, Utopia Foods, Inc. voluntarily expanded their original recall (first issued on December 13, 2022) to include their 200g packages of “Enoki Mushrooms”, imported from China, with clear and blue plastic packages with clear markings of “Best before 03/02/2023” or “Best before 03.09.23”. These products were distributed between January 6, 2023, and January 13, 2023, in NY, NJ, and CT, to wholesale companies for further distribution.
FDA’s investigation is ongoing to determine a potential source of contamination and whether any other products are contaminated or linked to illnesses. Additional information will be provided as it becomes available.
January 17, 2023
FDA, along with CDC and state and local partners, is investigating an outbreak of Listeria monocytogenes infections linked to enoki mushrooms. FDA has identified imported enoki mushrooms distributed by Utopia Foods, Inc. of Glendale, New York as a likely source of illnesses in this outbreak. Enoki mushrooms are long thin white mushrooms, usually sold in clusters. They are especially popular in East Asian cuisine and are also known as enokitake, golden needle, futu, seafood, or lily mushrooms.
As of November 17, 2022, CDC reports two illnesses included in this outbreak. Based on epidemiological information provided by CDC, both patients report consuming enoki mushrooms or eating at restaurants with menu items containing enoki mushrooms prior to becoming ill.
Through ongoing sampling efforts, FDA and state partners have been collecting and testing samples of enoki mushrooms. An import sample of enoki mushrooms branded as Utopia Foods, Inc. was collected by FDA and was reported as being positive for Listeria monocytogenes. Whole Genome Sequencing (WGS) analysis determined that the strain of Listeria found in this sample matches the strain of Listeria linked to illnesses in this outbreak.
As a result of this testing, Utopia Foods, Inc. voluntarily expanded their original recall (first issued on December 13, 2022) to include their 200g packages of “Enoki Mushrooms”, imported from China, with clear and blue plastic packages with clear markings of “Best before 03/02/2023” or “Best before 03.09.23”. These products were distributed between January 6, 2023, and January 13, 2023, in NY, NJ, and CT to wholesale companies for further distribution.
On December 13, 2022, as a result of earlier testing that determined enoki mushroom product was contaminated with Listeria monocytogenes, Utopia Foods Inc. issued a voluntary recall of 200g packages of “Enoki Mushrooms”, imported from China and shipped nationwide. The Listeria detected in their product at that time has not been linked to this outbreak or any reported illnesses.
FDA’s investigation is ongoing to determine a potential source of contamination and whether any other products are contaminated or linked to illnesses. Additional information will be provided as it becomes available.
Who to Contact
Consumers who have symptoms should contact their health care provider to report their symptoms and receive care.
To report a complaint or adverse event (illness or serious allergic reaction),
visit Industry and Consumer Assistance.
Follow us on X (formerly Twitter)