Laboratory Information Bulletin (LIB) 4303: Chloramphenicol in Crawfish Meat
Laboratory Information Bulletin
LIB No. 4303
Volume 19, No. 4, April 2003
LC/MS/MS Analysis of Chloramphenicol in Crawfish Meat
James S. Stuart, Heidi S. Rupp and Jeffery A. Hurlbut.
U.S. Food and Drug Administration, Pacific Regional Lab Northwest,
22201 23rd Drive SE, Bothell, WA 98021.
This Laboratory Information Bulletin (LIB) is a tool for the rapid dissemination of laboratory methods which appear to work. It does not necessarily report completed scientific work. Users must assure themselves by appropriate validation procedures that LIB methods and techniques are reliable and accurate for their intended use. Reference to any commercial materials, equipment, or processes does not in any way constitute approval, endorsement, or recommendation by the Food and Drug Administration.
Abstract
This method is an extension of LIB 4290 "LC/MS/MS Analysis of Chloramphenicol in Shrimp"1 by Barbara K. Neuhaus, Jeffrey A. Hurlbut, and Walter Hammack, to include the analysis of chloramphenicol in cooked crawfish. Crawfish meat is fortified at levels from 0.10 to 1.0 ppb of chloramphenicol, then extracted and analyzed using LIB 4290 as a guide. The sample preparation remains unchanged, but some extraction procedures have been slightly modified for expediency. Five replicate samples are analyzed at each of five fortification levels. Average absolute recoveries range from 109% for the lowest level to 98.1% for the highest level. The standard deviation ranges from 6.9% for the lowest level to 4.6%. Five matrix controls (unfortified crawfish meat) were also analyzed, in which no chloramphenicol was detected. The precursor ion is selected for m/z 321 and four daughter ions (m/z 152, 176, 194 and 257) are monitored for identification and assay. Determination was based on the least squares linear regression of the peak area of the m/z 152 daughter ion. For identification purposes, the ion ratios of each daughter ion versus the m/z 152 daughter ion of the fortified crawfish versus those of the chloramphenicol standards, agreed within 10% (relative) at chloramphenicol concentrations of 0.10-1.0 ppb.
Introduction
Chloramphenicol (CAP) is a broad spectrum antibiotic that was developed around 1950 and it has very effective antibacterial properties. Due to the unpredictable effects on patients, it has not been possible to identify a safe level of human exposure to chloramphenicol. This has lead to a zero tolerance policy in Europe, Canada, and now the United States.
Analytical methods for assaying chloramphenicol in shrimp have been available for a number of years, using derivatization of the analyte and GC/ECD for determination2. This GC/ECD method has an effective detection limit of five parts per billion and relies on chromatographic retention time for identification. The recent emergence of liquid chromatography/mass spectrometry (LC/MS) techniques capable of testing for CAP in complex matrices has a detection limit at or below one part per billion5. This method is capable of both quantitative and qualitative determination of CAP, and herein we are presenting a validation of the method for CAP in crawfish.
Reagents and Chemicals
Ethyl Acetate, n-Heptane, Methanol, and Acetonitrile (High Purity, HPLC, Residue Grade).
De-ionized Water (HPLC Grade).
Glacial Acetic Acid, Ammonium Acetate, and Sodium Chloride, Reagent Grade.
Chloramphenicol, USP Reference Standard (Lot N).
Diluent: 1:1 Methanol:Water made by mixing equal volumes of each solvent.
Mobile Phase A: 10mM Ammonium Acetate and 0.1% Acetic Acid in HPLC grade water. Two (2) liters of this solution is made by transferring 1.547g of ammonium acetate and 2.00 mL of glacial acetic acid to a 2000 mL volumetric flask and diluting to the mark with HPLC grade water.
Mobile Phase B: 95:5 Acetonitrile:Mobile Phase A. One (1) liter of this solution is made by adding 50mL of Mobile Phase A to a 1000mL volumetric flask and diluting to the mark with acetonitrile.
4% Sodium Chloride: (4% NaCl) to make one (1) liter of this solution transfer 40.0g of sodium chloride (NaCl) into a 1000mL volumetric flask and dilute to the mark with laboratory de-ionized water.
Apparatus
Instrument: Finnigan TSQ Mass Spectrometer with Surveyor High Performance Liquid Chromatograph / (LC/MS/MS) [See Instrument Parameters]
Chromatographic Column: Phenomenex LUNA 5µm C18 150 x 2mm
Food Processor: Robot-Coupe model RSI 2Y-1, or equivalent.
Centrifuge: Must be capable of holding 50mL centrifuge tubes and 3000 revolutions per minute (rpm).
Equipment: Wrist-Action Mechanical Shaker, Vortex Mixer, and Nitrogen Evaporator with heated water bath.
Aspiration Device: Fit a bored stopper in a trap flask. Connect arm of flask to vacuum source with vacuum hosing. Snugly insert a length of Teflon or flexible plastic tubing into the bore hole of the stopper. Attach a disposable pipettor tip to the "working end" of the tubing. This end is the snout that is used to suction off the heptane from the aqueous layer, and the tip can be changed between each sample. This device allows for the top layer of solvent to be aspirated into the flask when the snout tip is placed against the tube wall slightly above the liquid surface.
Centrifuge Tubes: Fifty milliliter (50mL) polypropylene, conical, with screw-caps.
Syringes: 1mL polypropylene for filtering extract.
Syringe Filters: 13 mm x 0.2µm PVDF (polyvinylidene fluoride) membrane filters.
Volumetric Glassware: Various class A pipettes and flasks.
Instrument Parameters
- Chromatography
- Gradient:
Minutes Mobile Phase A Mobile Phase B 0 100% 0% 15 20% 80% 15.5 100% 0% 20.5 100% 0% - Flow Rate: 200µL/minute
- Column Oven: 40°C
- Gradient:
- Autosampler Conditions
- Injection Volume: 10µL
- Syringe flush and wash volume: 6mL
- Sample Tray Temperature: 10°C
- Mass Spectrometry
- Ionization: Negative Ion Electrospray
- Spray Voltage: 1.5 kV
- Sheath Gas: N2 @ 80psi
- Capillary Temperature: 350°C
- Source Offset Voltage: 5 V
- Precursor Ion (Q1): m/z 321
- Collision Gas (Q2): Argon @ 2.5 milliTorr
- Collision Voltage: 26 V
- Product Ions (Q3): m/z 257, m/z 194, m/z 176, m/z 152
- Electron Multiplier Voltage: 1.27 kV
Sample Preparation
One hundred grams (100g) of headless, peeled and frozen crawfish and two hundred grams (200g) of dry ice are placed in the Robot-Coupe food processor. This mixture was then processed to a fine powder consistency. This mixture of powdered crawfish and dry ice is then de-gassed overnight in a freezer before proceeding. (There are a couple of safety reminders here: The mixture should not be stored in sealed containers, as the evolving gas will build up pressure presenting a possible bursting hazard. The second point is that depending on the total amount of dry ice involved, an asphyxiation hazard could develop in a walk-in freezer.) This dry-ice technique is based on the work of Bunch, et. al.3
Sample Extraction and Clean-up
Weigh ten grams (10g) of degassed, frozen crawfish powder into the first fifty milliliter (50mL) plastic centrifuge tube. Add fifteen milliliters (15mL) of ethyl acetate (EtOAc) to the centrifuge tube and cap tightly. Each tube needs to be shaken vigorously for ten minutes, a mechanical shaker is advisable. Each tube is then centrifuged for five minutes at 3000 RPM, and the supernatant is decanted into the second centrifuge tube. A second 15mL portion of EtOAc is added to the first sample tube. This tube is capped and vigorously shaken until the pellet of crawfish tissue at the bottom is completely broken up. The sample tube is centrifuged again for five minutes at 3000 RPM and the supernatant is decanted into the second centrifuge tube. This extraction is repeated a third time, for a total of forty five milliliters (45mL) of EtOAc extract in the second sample tube. The first sample tube with the crawfish pellet is now discarded (it is advisable to allow the tube to dry in a fume hood before disposal).
The extract in the second sample tube is reduced to dryness with a stream of nitrogen in a water bath at 45±5°C. At this time two milliliters of methanol is added to the second sample tube, capped and spun on the vortex mixer for about a minute. Twenty five milliliters (25mL) of 4% NaCl solution and twenty milliliters (20mL) of heptane are added to the second sample tube. This mixture is then vigorously shaken for thirty seconds, and then allowed to separate for several minutes until any emulsion breaks up. If the emulsion persists, it may be necessary to centrifuge the mixture lightly (1000rpm for a minute) to break it up. The top layer of heptane is removed and discarded. The de-fatting extraction is then repeated with another 20mL aliquot of heptane, and this too is removed and discarded.
The chloramphenicol is now extracted from the aqueous phase remaining in the second centrifuge tube, by adding fifteen milliliters (15mL) of EtOAc, capping tightly and shaking vigorously for about two (2) minutes. The mixture is then allowed to stand for several minutes until the upper organic phase is clear. It is important that all emulsion be broken before the organic phase is removed. It sometimes proves necessary to centrifuge lightly (about 1000rpm for a minute) to break up an especially stable emulsion. The organic phase is transferred to the third centrifuge tube and the extraction repeated twice more with 15mL aliquots of EtOAc. This final extract of forty five milliliters (45mL) is again reduced to dryness with a stream of nitrogen in a water bath at 45±5°C.
The dry residue is re-dissolved in a final volume of 1.00 mL of Diluent and this extract is transferred to a 1mL plastic syringe. The extract is then passed through a 0.2µm (PVDF) membrane filter into an auto-sampler vial. This extract is ready for analysis.
Standards
The primary stock standard was made by accurately weighing 20.1mg of USP Reference Standard Chloramphenicol to the nearest tenth of a milligram, and then diluting to 50.0 mL in methanol. This gives a standard of 402,000 ng/mL.
Working Standard 1 (WS1) is made by pipetting 1.00 mL of primary stock standard into a 100 mL volumetric flask and diluting to the mark with Diluent, giving a standard of 4020 ng/mL.
Working Standard 2 (WS2) is made by pipetting 1.00 mL of WS1 into a 100 mL volumetric flask and diluting to the mark with Diluent, giving a standard of 40.2 ng/mL.
The linearity/calibration standards were made according to the following table, by taking aliquots of WS2 and diluting to 10.0 mL, and then taking equal portions of the diluted standard and blank crawfish extract, mixing together, to give the final "tissue equivalent" standard. Blank crawfish extract consists of the final extract of unfortified crawfish, taken through the extraction method. To have enough of this blank crawfish extract on hand, one can elect to: extract multiple crawfish blanks; perform a scaled-up extraction of a larger aliquot of crawfish tissue; or create a reserve pool of previously extracted crawfish blanks or known-blank crawfish sample extracts. Blank crawfish extract is added to the plain standard CAP to help equalize any matrix effects in the collision induced fragmentation pattern.
| Aliquot of WS2 | Final Volume | Standard Concentration | Standard Concentration After 1:1 Dilution with crawfish extract | |
|---|---|---|---|---|
| A | 0.250 mL | 10.0 mL | 1.01 ppb | 0.503 ppb |
| B | 0.500 mL | 10.0 mL | 2.02 ppb | 1.01 ppb |
| C | 1.00 mL | 10.0 mL | 4.02 ppb | 2.02 ppb |
| D | 2.00 mL | 10.0 mL | 8.04 ppb | 4.02 ppb |
| E | 5.00 mL | 10.0 mL | 20.1 ppb | 10.1 ppb |
Sample Spiking and Method Design
Crawfish for analysis was retail, cooked, and frozen, crawfish meat imported from China. Individual 10 g samples were fortified to contain 0.10, 0.20, 0.30, 0.50, and 1.00 ppb CAP in crawfish, using 25, 50, 75, 125 and 250 µL of Working Standard 2 (WS2). Five (5) replicates of each of the five (5) fortification levels were analyzed. A blank crawfish sample is extracted with each of the fortification levels. This provided a population of n>30.
Since multiple samples were being handled concurrently, sample extraction generally took more than one day to perform. Partially extracted samples were held in a refrigerator (@5 ±2°C) overnight between extraction steps; and finished extracts may also be held in this same refrigerator prior to analysis.
For each day of instrumental analysis, four (4) sequential injections of the lowest calibration standard (Standard A) are made, followed by sequential injection of the calibration standards from lowest to highest. At least one blank crawfish extraction is processed with each extraction set, and the blank is analyzed before any of the fortified extracts. Between each set of five (5) replicates a calibration check is performed using the lowest calibration standard (Standard A).
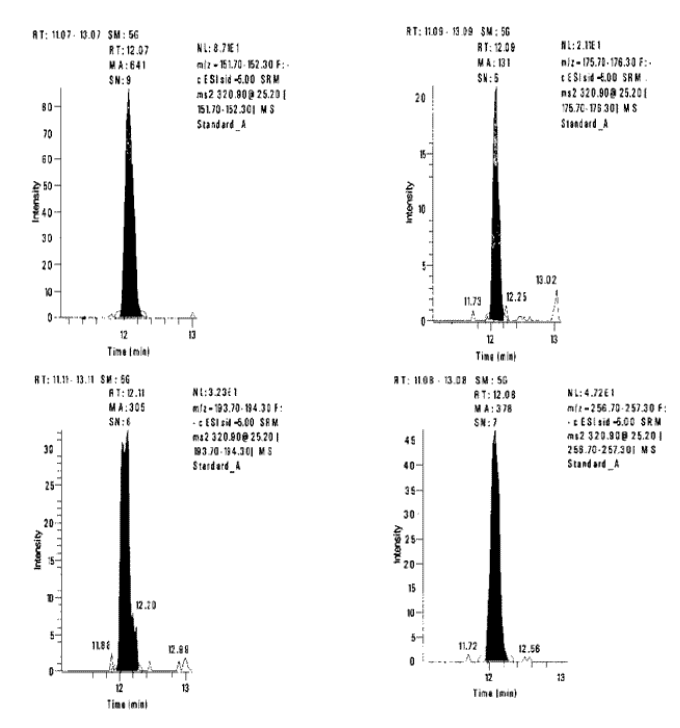
The LC/MS/MS analysis uses Selected Reaction Monitoring (SRM) to control the ions delivered to the detector. The first quadrupole is set to select the parent m/z 321 ion, the second quadrupole is where Collision Induced Dissociation (CID) occurs with argon gas, and the third quadrupole monitors four daughter ions (m/z 152, 176, 194 and 257). Quantitation is based on a least squares linear regression of the peak areas of the m/z 152 daughter ion, and the amount of chloramphenicol injected on the column in nanograms (ng).
Results and Discussion
Quantitation
After spiking, the actual concentration of chloramphenicol in the sample aliquot in picograms per gram depends on the weight of the individual sample aliquots in grams. This variability in concentration is normalized by comparing the percentage recovery of the individual samples. The average of five (5) recoveries of the m/z 152 ion is reported in Table 1, and these range from 109% for the lowest level of spiking to 98.1% for the highest. The Relative Standard Deviation for the recoveries ranged from 6.9% for the lowest level to 4.9% for the highest level.
Confirmation Chloramphenicol is identified by both chromatographic retention time and the ratio of the daughter ions (m/z 152, 176, 194, 257). Over the course of three analysis sets in as many weeks, the retention times for all monitored ions were predictable and consistent. For a single analysis set, the standard deviation of the retention time for any ion ranged from a high of 0.05 minute to a low of 0.03 minute. For all analyses the average value of the retention time for ion m/z 152 is 12.21 minutes, for all the other ions the value is 12.22 minutes. The standard deviation of these values is 0.14 minutes, giving a relative standard deviation of 1.16% for ion m/z 152, and 1.15% for all the other ions.
The product ions of Collision Induced Dissociation (CID) are employed to identify chloramphenicol by calculating the ratios of each daughter ion versus the m/z 152 daughter ion. The ratios of the fortified crawfish versus those of the chloramphenicol standards, agreed within 10% (relative) at chloramphenicol concentrations of 0.10-1.00 ng/g, [Table 2].
Conclusions
The method presented here is economical of both time and material. The scaled down liquid phase partitioning minimizes waste generation while applying a tried laboratory technique. The use of disposable centrifuge tubes, syringes, and filters keeps contamination problems to a minimum; this allows the method to exploit the great sensitivity of the instrument. It simultaneously provides reliable determination and confirmation of chloramphenicol in crawfish which is useful in a regulatory situation.
Acknowledgements
Many thanks to PRLNW chemist Rachel Tomlin for her friendly and efficient sample preparation. Thanks also to Barbara Neuhaus and Greg Mercer for their assistance with the LC/MS/MS.
References
- "LC/MS/MS Analysis of Chloramphenicol in Shrimp," Barbara K. Neuhaus, Jeffrey A. Hurlbut, and Walter Hammack. (2002) Laboratory Information Bulletin No. 4290.
- "Determination of Chloramphenicol Residues in Shrimp by Gas Chromatography with Electron Capture Detector" By Robert K. Munns, David C. Holland, Guy R. Stehly, Steven M. Plakas, Jose E Roybal, Jopseph M. Storey, and Austin R. Long. (1992) Laboratory Information Bulletin No. 3646.
- "Homogeneous Sample Preparation of Raw Shrimp with the Aid of Dry Ice," Elaine Bunch, Diane Altwein, Lloyd Johnson, Joyce Farley, and Amy Hammersmith. (1995) J. AOAC Int, 78, 883-887.
- "Principle and Practices of Method Validation" edited by A. Fajgelj and A. Ambrus; The Royal Society of Chemistry (2000)
- "Confirmation of Chloramphenicol Residue in Crawfish by Electrospray LC/MS," Allen Pfenning, Sherri Turnipseed, Jose Roybal, Mark Madson, Rebecca Lee, and Joseph Storey. (2002) Laboratory Information Bulletin No. 4294.
| Fortification Level | 0.100 ppb | 0.200 ppb | 0.300 ppb | 0.500 ppb | 1.00 ppb |
|---|---|---|---|---|---|
| Mean Recovery (n=5) | 109% | 102% | 99.8% | 98.4% | 98.1% |
| Relative Standard Deviation | 6.9% | 6.0% | 4.6% | 4.6% | 4.9% |
| ion ratio vs. m/z 152 | ||||
|---|---|---|---|---|
| Fortification Level* | m/z 257 ion | m/z 194 | m/z 176 | |
| 1.00 ppb | Average of five Standards | 0.575 | 0.479 | 0.238 |
| Average of five Samples | 0.561 | 0.454 | 0.237 | |
| Relative Percent Difference of Sample vs. Std. | 2.5% | 5.4% | 0.42% | |
| 0.500 ppb | Average of five Standards | 0.575 | 0.479 | 0.238 |
| Average of five Samples | 0.569 | 0.453 | 0.237 | |
| Relative Percent Difference of Sample vs. Std. | 1.0% | 5.6% | 0.42% | |
| 0.300 ppb | Average of five Standards | 0.570 | 0.492 | 0.231 |
| Average of five Samples | 0.609 | 0.471 | 0.239 | |
| Relative Percent Difference of Sample vs. Std. | 6.6% | 4.4% | 3.4% | |
| 0.200 ppb (1) | Average of five Standards | 0.570 | 0.492 | 0.231 |
| Average of five Samples | 0.575 | 0.474 | 0.248 | |
| Relative Percent Difference of Sample vs. Std. | 0.87% | 3.7% | 7.1% | |
| 0.100 ppb (2) | Average of five Standards | 0.565 | 0.471 | 0.232 |
| Average of five Samples | 0.578 | 0.469 | 0.256 | |
| Relative Percent Difference of Sample vs. Std. | 2.3% | 0.43% | 9.8% | |
Figure 1.
Ion Chromatograms of the Daughter Ions for Standard A