Laboratory Information Bulletin (LIB) 4290: Chloramphenicol in Shrimp
LC/MS/MS Analysis of Chloramphenicol in Shrimp
Volume 18, No. 9, September 2002
Barbara K. Neuhaus,* Jeffrey A. Hurlbut* and Walter Hammack**
* Food & Drug Administration, Pacific Regional Lab - NW, 22201 23RD Drive SE, Bothell, WA 98021
** Chemical Residue Lab, FL Dept. of Agriculture & Consumer Services, 3125 Conner Blvd., Tallahassee, FL 32399
This Laboratory Information Bulletin (LIB) is a tool for the rapid dissemination of laboratory methods which appear to work. It does not necessarily report completed scientific work. Users must assure themselves by appropriate validation procedures that LIB methods and techniques are reliable and accurate for their intended use. Reference to any commercial materials, equipment, or processes does not in any way constitute approval, endorsement, or recommendation by the Food and Drug Administration.
Abstract
Recently our laboratory (FDA, PRL-NW) was given the task of testing the performance of a method developed at the Chemical Residue Lab of the Florida Dept. of Agriculture. This is a liquid chromatographic mass spectrometric (LC/MS/MS) method for qualitative and quantitative detection of chloramphenicol (CAP) in shrimp at the sub parts per billion (ppb) level. Shrimp is pulverized with dry ice, is extracted with ethyl acetate, evaporated with N2, treated with hexane/aqueous NaCl, extracted back into ethyl acetate, dissolved into methanol-water after evaporation, and injected into an LC/MS. CAP eluted from the C18 LC column at about 12.2 min using an acetic acid - ammonium acetate - acetonitrile - water mobile phase. The mass spectrometer was operated in the negative ion mode using selected reaction monitoring, and the precursor ion at m/z = 321 yielded four main product ions of m/z = 257, 194, 176 and 152. The peak area of the m/z 152 peak was used for quantitation. Linear plots were obtained between 0.50 and 10.0 ng/mL CAP. Shrimp tissues were fortified with CAP at 0.10, 0.25, 0.50 and 1.0 ng/mL. Overall recoveries were 85, 92, 85 and 102 % with % RSD values of 9.4, 1.6, 3.1 and 2.5% respectively. The limit of quantitation (LOQ) was 0.3 ng CAP per g of shrimp (0.3 ppb), and the limit of detection (LOD) was estimated to be 0.08 ppb.
Introduction
Chloramphenicol (CAP) was first isolated from Streptomyces venezuelae in the 1940's (1,2), is a wide range antibiotic which interferes with protein synthesis of many gram-negative and gram-positive bacteria (3), and has toxic effects on humans (4). Miscellaneous toxic effects are due to the dichloride carbon alpha to the carbonyl group; this carbon readily undergoes substitution with nucleophiles such as those found on proteins (5). See the figure below.
The main potential human toxicity is depression of red blood cell production in bone marrow leading to aplastic anemia (3,6). In spite of its potential toxicity, CAP is sometimes used at therapeutic doses for treatment of serious infections in humans; however, it has not been possible to identify a safe level of human exposure to CAP. Because of the unpredictable effects of dose on different patient populations, Federal regulations prohibit its use in food producing animals and animal feed products (7).
Even though use of CAP in meat producing animals and aquaculture is banned in the European Union (EU), Canada and United States, illegal use of CAP to treat seafood products remains a possibility due to its broad spectrum activity, ready availability and low cost (8). Both the EU and Canada have recently detected low levels of CAP in imported shrimp from China, Thailand and Vietnam (7,9,10). Canada's and EU's present methodology allow detection of CAP at 2.5 and 0.3 ppb respectively (7,11). The official method used by the U.S. detects CAP in shrimp at the 5 ppb level (7); however, modifications and new methods should lower our detection limit to at or below 1 ppb (7).
As of September 2002, there were over 24,000 references to CAP in the scientific literature with 4000 of these dealing with detection; however, only a few of these references dealt with detection of CAP in aquaculture. A common approach to the analysis of CAP in seafood tissues is first cleanup utilizing liquid/liquid extraction and solid phase extraction (SPE) followed by derivatization to form volatile derivatives, and analysis by GC/Electron Capture Detection (GC-ECD) (8, 12). Munns GC-ECD method gave a detection limit of 1 ppb in shrimp, and the lowest spike level used by the confirming laboratories was 5 ppb (12). Liquid Chromatography (LC) using UV detection was also commonly used (13 - 19) which gave detection and quantitation limits at 5 and 10 ppb respectively in aquaculture tissue (13). GC using mass spectrometry (MS) detection was used in several publications (20-22) with detection limits at about 1 ppb. There a few papers involving LC-MS separation and semi-quantitative detection of CAP in aquaculture (23-25) with a detection limit in shrimp at 0.5 ppb (24). LC-MS methods for separation and detection of CAP appear to have the best potential for both low detection levels and strong conformational information. Several additional CAP LC-MS procedures for matrices other than seafood tissue are reported (26 - 31). At least two enzyme linked immunosorbent assay (ELISA) kits have recently been developed (Veratox and Ridascreen) which claim to be able to detect CAP in seafood tissue in the ppt to low ppb region (32, 33); however, results using these kits have not been published. An additional aspect of shrimp sample preparation using dry ice was presented in a publication by Bunch, et. al. (34).
The LC/MS/MS method described here allows for quantitative detection of CAP in shrimp at the sub-ppb level, allows for qualitative confirmation of the presence of CAP in shrimp, and does not require the use of an internal standard.
Chemicals, Equipment & Conditions
Reagents
- Ethyl Acetate: High Purity; VWR Catalog No. BJ099-4
- N-Hexane: Chrom AR HPLC Grade; VWR Catalog No. MK516706
- Methanol: HPLC Grade; JT Baker
- Acetonitrile: HPLC Grade; JT Baker
- Water: Generated from Millipore Milli-Q Plus - Ultra Pure Water System
- Glacial Acetic Acid: Sigma ACS Reagent Grade; Catalog No. A-0808
- Ammonium Acetate: Sigma Ultra Grade; Catalog No. A-7330
- Sodium Chloride: Spectrum Chemical Mfg. Corp; Catalog No. S1240
- Sodium Sulfate: Aldrich ACS Reagent Grade; Catalog No. 23,931-3
- Chloramphenicol: USP Reference Standard; Lot N
- Diluent: 1:1 Methanol:Ultrapure Water
Equipment
- LC: ThermoQuest Surveyor MS Pump and autosampler
- MS Detector: ThermoQuest Finnigan TSQ 7000 with API2 used in electrospray mode with metal needle option (API-2 metal needle kit; part no. OPTON-53003 from ThermoFinnigan).
- Instrument software:
Xcalibur Home Page, Version 1.2;
Surveyor AS, Version 1.2 SP 1;
Surveyor MS pump, Version 1.2;
TSQ MS, Version 1.1 - LC Column: Phenomenex LUNA 5 µm C18 150 x 2 mm
- Robot Coupe model R10
- Centrifuge capable of holding 50 mL centrifuge tubes and spinning at 3000 rpm
- Mechanical shaker: Burrell Wrist Action Shaker
(Arms are rotated so tubes are shaken in a horizontal position.) - Vortex mixer: Vortex Jr. Mixer from American Scientific Products, catalog no. S82251-1
- N-EVAP: Organomation N-EVAP Analytical Evaporator
- Sample Tubes: 50 mL polypropylene conical centrifuge tubes ("BLUE MAX"),
sterile, 30 x 115 mm, catalog no. 21008-940 from VWR - Micropipettors: Wheaton Calibra Digital Micropipettors;
10-100 µL (catalog no. 851164), 100-1000 µL (catalog no. 851168).
A calibration check was performed with Diluent mass measurements before use. - Pipet Tips: 10-100 µL, yellow, polypropylene, catalog no. 851272 from Wheaton;
100-1000 µL, blue, polypropylene, catalog no. 100201 from Sorenson BioScience - Cotton Tipped Applicators: Puritan cotton tipped applicators with wooden handles
(The wooden end is used to loosen the composite after centrifuging.) - 125 mL Glass Separatory Funnels
- 8 cm Glass Funnels
- Glass Wool: Corning Fiberglass 8 µm
(This is washed with acetone and air dried after being received into our lab.) - Syringe Filters: Whatman 4 mm PVDF syringe filters with tube tips,
0.2 µm, catalog no. 28137-512 from VWR - 1 mL Disposable Syringes: catalog no. BD309602 from VWR
- Snap-Cap Sample Tubes: 0.5 mL polypropylene tubes with attached cap,
catalog no. 1605-0099 from USA/Scientific - Volumetric Pipets & Flasks: Class A
Standards
Calibration and spike standards were prepared in 50:50 Methanol : Ultra Pure Water (Diluent) from USP CAP. Preparation of a typical set of standards is given below.
- 37.1 mg CAP dissolved in 50.0 mL methanol to yield 742,000 ng/mL (#1)
(note: the following standards were diluted with Diluent) - 3.00 mL #1 to 100.0 mL to yield 22,300 ng/mL (#2)
- 3.00 mL #2 to 50.0 mL to yield 1,340 ng/mL (#3)
- 3.00 mL #3 to 100.0 mL to yield 40. ng/mL
- The 40. ng/mL standard was used directly and diluted to yield six standards 1.0 through 40 ng/mL. Each of the six was again diluted 1:1 with unspiked sample matrix to yield CAP concentrations from 0.50 to 20. ng/mL; these were used for the calibration curves. The concentration of shrimp in the final dilution of each standard was 10 g/mL.
Spiked Samples
Spike recoveries were performed at CAP concentrations of 0.10, 0.25, 0.50, and 1.0 ppb in the shrimp. Five recoveries at each level were run along with both a reagent and a sample blank. The spike was added to the shrimp before extraction in step (b) of the extraction-cleanup procedure.
LC Conditions
| Mobile Phase: A -- | 0.1% acetic acid and 10 mM ammonium acetate in water | ||
| B -- | 0.1% acetic acid and 10 mM ammonium acetate in 95:5 CH3CN:Water | ||
| Gradient: | Minutes | %A | %B |
|---|---|---|---|
| 0 | 100 | 0 | |
| 15 | 20 | 80 | |
| 15.5 | 100 | 0 | |
| 20.5 | 100 | 0 | |
Flow Rate: 200 µL/min
Column Temperature: 40°C
Autosampler Conditions
| Autosampler: | Injection Volume - 10 µL (no waste injection) |
| Syringe Flush Volume and Wash Volume - 6 mL | |
| Sample Tray Temperature - 10 °C |
Mass Spectrometer Conditions
Mode: Negative Ion Electrospray - with metal needle option. The metal needle was connected with a zero dead volume union to the fused silica capillary delivering the mobile phase to the ESI source; this eliminated the frequent need to reposition the capillary. Note that the parameters need to be optimized for each instrument. The following conditions were found to maximize response on our instrument.
| Precursor Ion (m/z): | 321 |
| Product Ions (m/z): | 257, 194, 176, 152 |
| Spray Voltage: | 1.5 kV |
|---|---|
| Collision Voltage: | 26 V |
| Source Offset Voltage: | 5 V |
| Electron Multiplier Voltage: | 1.27 kV |
| Capillary Temperature: | 350 °C |
| N2 Sheath Gas: | 80 Arbitrary Units |
| N2 Auxiliary Gas: | 35 psi |
| Collision Gas: | Ar |
Method
Sample Preparation
A block of frozen shrimp was partially thawed, separated, and re-frozen. A mass of dry ice which corresponded to between 100 and 200% of the mass of the shrimp was broken into small pieces with a hammer. About half of the dry ice was ground to a fine powder in the Robot Coupe, and then the remaining dry ice was added and ground to a fine powder. The frozen shrimp was added slowly and ground until the shrimp had been reduced to a uniform powder. The ground shrimp was transferred to loosely covered plastic containers and placed in the freezer, allowing the CO2 to sublime. Note that the tails, fins and shells of the shrimp take up 20% of the mass (34). If these parts are not removed then 12.5 g of complete shrimp correspond to 10. g of cleaned shrimp.
Extraction and Cleanup
Note: four sample tubes denoted by numbers #1 - #4 are used for each sample.
- Check sample tube #1 for leakage by adding an aliquot of ethyl acetate, capping and shaking. Discard the ethyl acetate. Do not use tubes that leak.
- Weigh 12.5 g ground shrimp into sample tube #1. (This assumes shrimp with tails, fins and shells are used which corresponds to 10. g of cleaned shrimp). Add 20 mL ethyl acetate. Let sit at room temperature for about 15 min.; this will prevent leakage during the shaking step caused by an increase in pressure due to warming.
- Shake for 10 minutes on the mechanical shaker. (Arms are rotated so the tubes are held in a horizontal position.)
- Centrifuge at 3000 rpm for 5 min. and decant ethyl acetate into sample tube #2.
- Add 20 mL ethyl acetate to sample tube #1 containing the previously centrifuged ground shrimp. Use the wooden end of a disposable cotton tip applicator to break up the ground shrimp. Cap and shake the tube for a few seconds, then centrifuge at 3000 rpm for 5 min. Decant the ethyl acetate into sample tube #2.
- Place sample tube #2 on the N-EVAP in water bath at approximately 45°C and evaporate to about 0.5 mL. Transfer to sample tube #3 with two 2 mL portions of ethyl acetate using a Pasteur pipet (being sure to wash down the sides of the tube to remove any residue). Do not transfer the undissolved material in the bottom of the tube.
- Place sample tube #3 containing about 4.5 mL sample solution on the N-EVAP. During evaporation lower the carousel periodically into the 45°C water bath; for the last 0.5 mL evaporate at room temperature just to dryness.
- Add 2 mL methanol to sample tube #3; vortex for 30 sec.
- Add 4% NaCl solution (12 g NaCl/300 mL water) to the methanol solution in sample tube #3 up to the 25 mL mark. Cap and shake for about 5 sec.
- Transfer the contents of sample tube #3 to a 125 mL separatory funnel.
- Add 20 mL hexane to sample tube #3; cap and shake for about 5 sec. Transfer to the same separatory funnel containing the aqueous portion. Stopper and shake the separatory funnel for 30 sec.; vent as needed.
- Drain the bottom layer (aqueous portion) back into sample tube #3; discard the hexane layer.
- Repeat steps j - l.
- Transfer the contents of sample tube #3 to the separatory funnel.
- Add 15 mL ethyl acetate to sample tube #3, cap and shake for about 5 sec. Transfer to the separatory funnel containing the aqueous portion. Stopper and shake the separatory funnel for about 30 sec.
- Drain the bottom layer (aqueous portion) back into sample tube #3. Drain the ethyl acetate layer through about 10 g Na2S04 (held in a glass funnel with glass wool and previously rinsed with about 5 mL ethyl acetate) into sample tube #4.
- Repeat steps n - p.
- Rinse the Na2S04 with about 5 mL ethyl acetate.
- Place sample tube #4 on the N-EVAP in a 45 °C water bath and Evaporate to about 0.5 mL.
- Wash down the sides with 2 mL ethyl acetate to remove any residue. Continue evaporating with periodic warming by lowering the carousel into the 45°C water bath; evaporate the last 0.5 mL at room temperature just to dryness.
- Add 0.50 mL Diluent to sample tube #4 and vortex for 30 sec.
- Filter through 0.2 µm PVDF syringe filter into a snap cap sample tube. This was diluted 1:1 with either Diluent or with standards and injected. Note that all solutions injected corresponded to 10 g shrimp/mL. This sample is now ready for LC/MS.
Results and Discussion
CAP eluted from the LC at about 12.2 min. The mass spectrometer was operated in the negative ion mode using selected reaction monitoring, and the precursor ion at m/z = 321 yielded four main product ions of m/z = 257, 194, 176 and 152. This is similar to the MS2 results observed by Pfenning, by Turnipseed, and by Li (24, 25, 26, 31). Possible identities of these four ions are given in Table 1.
| m/z | Possible Identity using 35Cl |
|---|---|
| 321 | [M-H]- |
| 257 | [M-H - (HCOCl)]- |
| 194 | [M-H - (NH2COCHCl2)]- |
| 176 | [m/z 194 - (H2O)]- |
| 152 | [O2N-C6H4-CHOH]- |
Under our conditions, the m/z 152 product was the base peak, and it was used for quantitation. The ranges in ion peak area ratios for multiple injections of standards and spikes, expressed as peak area % of the 152 ion peak are given in Table 2.
| m/z | 257 | 194 | 176 |
|---|---|---|---|
| Range | 49-64 % | 29-37 % | 23-33 % |
| Average | 55 % | 34 % | 28 % |
| % RSD | 4.1 % | 4.8 % | 6.3 % |
Standards were run from 0.50 to 20.0 ng/mL (which correspond to 0.05 to 2.0 ppb CAP in shrimp), and the four product ions were scanned over 0.25 sec. The m/z 152 peak was chosen for performing the quantitative analysis. Plots were made of m/z 152 peak area versus ng/mL CAP. These calibration standards were run at the beginning of an injection sequence. At the end of each sequence, a standard was again injected. The response of the reinjected standard had to be within 20% of the initial response.
The calibration curves were linear from 0.50 through 10.0 ng/mL CAP yielding correlation coefficients (r) of between 0.9979 and 0.9999 (average r = 0.9992) for three separate runs. The curves deviated from linearity above 10. ng/mL but gave a nice quadratic fit for standards between 0.5 and 20. ng/mL with an average quadratic regression (r) value of 0.9998. Some have suggested using the quadratic fit for calculations in the non-linear region (35); however, all data reported was obtained from the linear equations. Five standards between 0.50 and 20.0 ng/mL were run for the 0.10 and 0.25 ppb spikes, and four standards between 1.0 and 20.0 ng/mL were run for the 0.50 and 1.0 ppb spikes. In each case the upper 20.0 ng/mL standard was dropped for the linear plots.
Several portions (12.4 g - 12.7 g) of ground whole shrimp were spiked at 0.10, 0.25, 0.50, and 1.0 ppb levels and then taken through the cleanup and analysis procedure. Five repetitions of each spike were made. The recovery data is given in Table 3. Average recoveries ranged from 85 % at the 0.10 ppb spike level to 102 % at the 1.0 ppb spike level with an overall recovery for the twenty runs of 91 %. The corresponding % RSD values ranged from 9.4 % at the 0.10 ppb spike level to 2.5 % at the 1.0 ppb level.
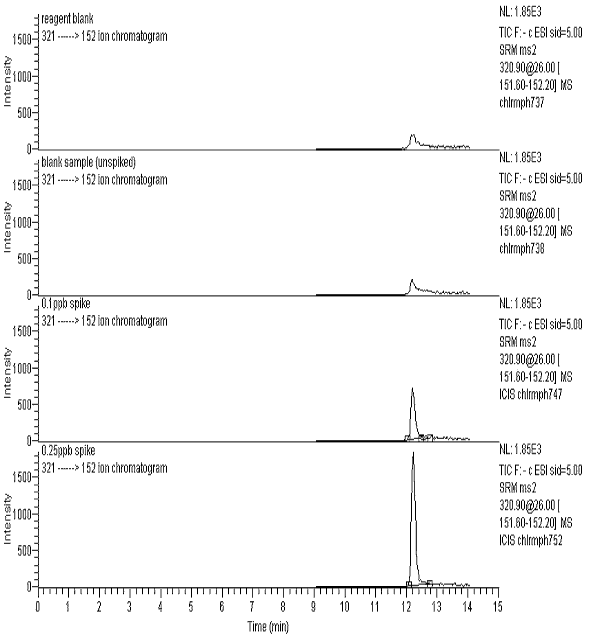
Reagent blanks and unspiked blank samples exhibited an interference at the CAP retention time. The average 152 ion peak height was 150 for the reagent blank and 213 for the unspiked blank samples. See Figure 1. A signal of 213 was taken as the noise level, and the average S/N values for the 0.1, 0.25 and 0.5 ppb recoveries are given in Table 4. These table values indicate that a CAP concentration of 0.08 ppb would yield a S/N = 3 (LOD), and a concentration of 0.3 ppb would yield a S/N = 10 (LOQ).
| Run # | 0.10 ppb | 0.25 ppb | 0.50 ppb | 1.0 ppb |
|---|---|---|---|---|
| 1 | 93 % | 92 % | 88 % | 101 % |
| 2 | 83 | 90 | 88 | 107 |
| 3 | 85 | 91 | 85 | 100 |
| 4 | 91 | 91 | 84 | 102 |
| 5 | 73 | 94 | 82 | 101 |
| Average: | 85 | 92 | 85 | 102 |
| % RSD | 9.4 | 1.6 | 3.1 | 2.5 |
| Recovery Sample | Ave Peak Height from m/z 152 Ion Chromatograms |
Ave S/N |
|---|---|---|
| 0.1 ppb | 826 | 3.9 |
| 0.25 ppb | 1884 | 8.8 |
| 0.5 ppb | 3487 | 16.4 |
Under our conditions, the extraction, cleanup and LC/MS/MS determination gave a detection limit of 0.08 ppb and a limit of quantitation of 0.3 ppb CAP in shrimp. The method also allowed for analysis without the aid of an internal standard, and it allowed for the confirmation of CAP from the precursor and product ions. See Figure 2 for examples of the ion chromatograms of the four product ions.
Acknowledgements
We want to thank William Chase Jr., Dr. Greg Mercer, Dr. Sherri Turnipseed, and Dr. Mary Carson for their technical assistance.
References
- 2120, The Merck Index, 12th Edition, 1996.
- Q. R. Bartz, "Isolation and Characterization of Chloromycetin" (1948) J. Biol. Chem. 172, 445-450
- www.encyclopedia.com/html/cl/chloramp.asp
- J. E. Roybal, "Chloramphenicol and Related Drugs," Analytical Procedures for Drug Residues in Food of Animal Origin, (1998) Eds. S. B. Turnipseed and A. R. Long, Science Technology System, Sacramento, CA, pp. 227-260
- B. R. Baker, Design of Active-Site-Directed Irreversible Enzyme Inhibitors. The Organic Chemistry of the Enzymatic Active-Site, John Wiley and Sons, New York, 1967.
- S. B. Clark, R. A. Barrell, J. M. Nandrea, C. A. Geisler and J. A. Hurlbut, "Determination of Chloramphenicol Residue in Raw Milk by Gas-Liquid Chromatography with Electron-Capture Detector" (1991) Laboratory Information Bulletin, 3529, 8 pages
- FDA News, June 14, 2002;http://www.fda.gov/bbs/topics/NEWS/2002/New00815.html
- A. P. Pfenning, J. E. Roybal, H. S. Rupp, S. B. Turnipseed, S. A. Gonzales and J. A. Hurlbut, "Simultaneous Determination of Residues of Chloramphenicol, Florfenicol, Florfenicol Amine, and Thiamphenicol in Shrimp Tissue by Gas Chromatography with Electron Capture Detection" (2000) J. AOAC Int. 83 (1), 26-30
- FDA Veterinarian Newsletter, Volume XVII, No. IV, July/August 2002; http://www.fda.gov/cvm/July_August.htm
- http://www.mdac.state.ms.us/Library/AgencyInfo/PressReleases/Shrimp7-24-2002.pdf
- http://www.seafood.com/news/current/68213.html
- R. K. Munns, D. C. Holland, J. E. Roybal, J. M. Storey, A. R. Long, G. R. Stehly, and S. M. Plakas, "Gas Chromatographic Determination of Chloramphenicol Residues in Shrimp: Interlaboratory Study" (1994) J. AOAC Int. 77 (3) 596-601
- A. E. Tyrpenou, G. G. Rigos and F. Athanassopoulou, "Determination of Chloramphenicol Residues in Gilthead Seabream (Sparus aurata L.) Tissues by HPLC-PDA" (2002) J. Liq. Chromatogr. Rel. Technol. 25 (4) 655-663
- Way-Shyan Wang, Tain-Yao Hsu, Shu-Peng Ho, Sheng-Tsung Chang and Chin-Wen Shih, "Simultaneous Determination of Chloramphenicol, Florfenicol and Thiamphenicol in Tilapia Tissues by HPLC" (2001) J. Chinese Soc. Vet. Sci., 27 (3) 22-231
- C. Hummert, B. Luckas and H. Siebenlist, "Determination of Chloramphenicol in Animal Tissue Using High-Performance Liquid Chromatography with a Column-Switching System and Ultraviolet Detection" (1995) J. Chromatogr. B Biomed. Appl. 668 (1) 53-58.
- S. Y. Lin and S. Y. Chiu, "High Performance Liquid Chromatographic Determination of Chloramphenicol in Prawn and Eel Feeds" (1990) J. Chinese Soc. Vet. Sci., 16 (4) 281-285
- J. M. Degroodt, B. W. De Bukanski, J. De Groof, H. Beernaert and S. Srebrnik, "Chloramphenicol and Nitrofuran Residue Analysis by HPLC and Photodiode Array Detection in Meat and Fish" (1992) J. Liq. Chromatogr. 15 (13), 2355-2371
- T. Nagata and M. Saeki, "Simultaneous Determination of Thiamphenicol, Florfenicol and Chloramphenicol Residues in Muscles of Animals and Cultured Fish by Liquid Chromatography" (1992) J. Liq. Chromatogr. 15 (12) 2045-2056
- M. Horie, Y. Hoshino, N. Nose, H. Iwasaki and H. Nakazawa, "Simultaneous Determination of Antibiotics and Synthetic Antibacterials in Fish by High Performance Liquid Chromatography" (1985) Eisei Kagaku 31 (6) 371-376
- T. Nagata and H. Oka, "Detection of Residual Chloramphenicol, Florfenicol, and Thiamphenicol in Yellowtail Fish Muscles by Capillary Gas Chromatography-Mass Spectrometry" (1996) J. Agric. Food Chem., 44 (5) 1280-1284
- B. A. Aladar, S. M. Plakas and A. R. Long, "Optimization of the Analytical Performance of the Magnetic Sector Mass Spectrometer for the Identification of Residual Chloramphenicol in Shrimp" (1994) Biol. Mass Spectrom. 23 (11) 665-674
- M.-F. Pochard, G. Burger, M Chevalier and E. Gleizes, "Determination of Chloramphenicol Residues by Reversed-Phase High-Performance Liquid Chromatography Application to a Pharmacokinetic Study in Rainbow Trout with Confirmation by Mass Spectrometry" (1987) J. Chromatogr. 409 315-324
- E. D. Ramsey, D. E. Games, J. R. Startin, C. Crews and J. Gilbert, "Detection of Residues of Chloramphenicol in Crude Extracts of Fish and Milk by Tandem Mass Spectrometry" (1989) Biomed. Environ. Mass Spectrom. 18 (1) 5-11
- A. Pfenning, S. Turnipseed, J. Roybal, C. Burns, M. Madson, J. Storey and R. Lee, "Confirmation of Multiple Phenicol Residues in Shrimp by Electrospray LC/MS" (2002) Laboratory Information Bulletin 4284 13 pages.
- A. Pfenning, S. Turnipseed, J. Roybal, C. Burns, M. Madson, J. Storey and R. Lee, "Confirmation of Chloramphenicol Residues in Crawfish by Electrospray LC/MS" (2002) Laboratory Information Bulletin Submitted for Publication, 12 pages.
- T. L. Li, Y. J. Chung-Wang and Y. C. Shih, "Determination and Confirmation of Chloramphenicol Residues in Swine Muscle and Liver" (2002) J. Food Sci., 67 (1) 21-28
- V. Hormazabal and M. Yndestad, "Simultaneous Determination of Chloramphenicol and Ketoprofen in Meat and Milk and Chloramphenicol in Egg, Honey, and Urine Using Liquid Chromatography-Mass Spectrometry" (2001) J. Liq. Chromatog. Rel. Technol. 24 (16) 2477-2486
- R. Hirsch, T. A. Ternes, K. Haberer, A. Mehlich, F. Ballwanz and K-L Kratz, "Determination of Antibiotics in Different Water Compartments via Liquid Chromatography-Electrospray Tandem Mass Spectrometry" (1998) J. Chromatog. A 815 (2) 213-223
- M. H. Akhtar, K. A. Elsooud, A. M. Shehata and Anwar-Ul-Haq, "Fate and Residues of 14C-Chloramphenicol in Laying Chickens" (1996) J Environ. Sci. Health Pt. B Pesticides Food Contamin. Agricult. Wastes 31 (5) 1061-1084
- B. Delepine and P. Sanders, "Determination of Chloramphenicol in Muscle Using a Particle Beam Interface for Combining Liquid Chromatography with Negative-Ion Chemical Ionization Mass Spectrometry" (1992) J. Chromatog. Biomed. Appl. 582 (1-2) 113-121
- S. Turnipseed, C. Burns, J. Storey, R. Lee and A. Pfenning, "Confirmation of Multiple Phenicol Residues in Honey by Electrospray LC/MS" (2002) Laboratory Information Bulletin, 4281 12 pages
- Vetatox for Chloramphenicol, Neogen Corp., 620 Lesher Place, Lansing MI 48912; http://www.neogen.com/verachloramp.htm
- RidaScreen Chloramphenicol; http://www.r-biopharm.com/general/start.php
- E. A. Bunch, D. M. Altwein, L. E. Johnson, J. R. Farley and A. A. Hammersmith, "Homogeneous Sample Preparation of Raw Shrimp Using Dry Ice" (1995) J. AOAC Int. 78 (3) 883-887
- D. Bylund, "Chemometric Tools for Enhanced Performance in Liquid Chromatography - Mass Spectrometry," (2001) Ph. D. Thesis, Uppsala University, Pg 28.
Figure 1 - m/z 152 Ion Chromatograms
Figure 2 - Ion Chromatograms for Four Product Ions
With a 0.5 ppb CAP Spike