Laboratory Information Bulletin (LIB) 4302: Chloramphenicol in Crab Meat
LC/MS/MS Analysis of Chloramphenicol in Crab Meat
Volume 19, No. 4, April 2003
Heidi S. Rupp, James S. Stuart and Jeffrey A. Hurlbut
U.S. Food and Drug Administration, Pacific Regional Lab Northwest,
22201 23rd Drive SE, Bothell, WA 98021.
This Laboratory Information Bulletin (LIB) is a tool for the rapid dissemination of laboratory methods which appear to work. It does not necessarily report completed scientific work. Users must assure themselves by appropriate validation procedures that LIB methods and techniques are reliable and accurate for their intended use. Reference to any commercial materials, equipment, or processes does not in any way constitute approval, endorsement, or recommendation by the Food and Drug Administration.
Abstract
An LC/MS/MS method is described for the determination and confirmation of chloramphenicol (CAP) in cooked crab meat. It is an extension of another PRLNW-developed LC/MS/MS method (1, 2) for quantitative detection of chloramphenicol in shrimp at the low ppb level. The method involves: pulverization of cooked crab meat with dry ice; extraction of the CAP into ethyl acetate (EtOAc); evaporation (N2) of the EtOAc; addition of methanol, aqueous sodium chloride, and heptane; extraction of the lipids into the heptane, followed by extraction of the aqueous phase with EtOAc; evaporation (N2) of the EtOAc; dissolution into methanol-water; filtration; and separation/detection/confirmation using LC/MS/MS. Crab meat was fortified at 0.10 (1st), 0.10 (2nd), 0.25, 0.50, and 1.0 ng/g (ppb) chloramphenicol. Average absolute recoveries were 53, 51, 67, 84, and 86% respectively, with RSD's all less than 1%. Four daughter ions (m/z 152, 176, 194 and 257) were monitored off of the m/z 321 precursor ion. Determination was based on a standard curve using the peak areas of the m/z 152 daughter ion (the base peak), for standard solutions equivalent to 0.10, 0.20, 0.50, and 1.0 ppb in tissue (made with control crab extract). A set of six matrix controls (unfortified crab meat) were also analyzed, in which no chloramphenicol was detected. For identification purposes, the ion ratios (of each daughter ion versus the base daughter ion) of the fortified crab versus those of the chloramphenicol standards, agreed within 10% (relative) at chloramphenicol concentrations of 0.25-1.0 ppb, but increased to up to 21% for the m/z 257 ion in one set of the 0.10 ppb fortified crab.
Introduction
Chloramphenicol (CAP) is a broad spectrum antibiotic that was developed around 1950 and it has very effective antibacterial properties. Several years of clinical use has produced a significant amount of evidence relative to the toxic effects of chloramphenicol on humans. There is a potential for misuse in domestic and international markets because chloramphenicol is effective in animal therapy, including aquaculture species. Chloramphenicol residue may be present in the tissue of treated food producing animals thereby posing a health risk to some consumers. Consequently, FDA does not permit its use in food producing animals. Antibiotic residues in food are a global public health concern because they help to promote the evolution of bacteria to become resistant to many of our antibiotics.
Analytical methods for CAP in shrimp have been available for a number of years for GC/MS.(3, 4) Recently, the FDA has developed LC/MS methods for CAP in shrimp1 and CAP in crab.(5) Although the latter crab method is specific for our analyte, we found that the former shrimp method is easier to perform, gives slightly better recoveries, and makes use of triple quadrupole technology which allows for daughter ion identification and is more robust than ion trap methodologies. We have further refined the shrimp method to make it more streamlined and safer by using multiple centrifuge tubes instead of separatory funnels, and by using heptane instead of hexane (to minimize exposure to neurotoxic solvents).
Reagents and Chemicals
Ethyl Acetate, N-Heptane, Methanol, and Acetonitrile (High Purity, HPLC, Residue Grade).
Glacial Acetic Acid, Ammonium Acetate, and Sodium Chloride, Reagent Grade.
Chloramphenicol, USP Reference Standard (Lot N).
De-ionized Water (needs to be filtered when used for HPLC).
Diluent: 1:1 Methanol:Water made by mixing equal volumes of each solvent.
Mobile Phase A: 10mM Ammonium Acetate and 0.1% Acetic Acid in HPLC grade water. Two (2) liters of this solution is made by placing 1.547g of ammonium acetate and 2.00 mL of glacial acetic acid into a 2000 mL volumetric flask and diluting to the mark with HPLC grade water.
Mobile Phase B: 95:5 Acetonitrile:Mobile Phase A. One (1) liter of this solution is made by adding 50mL of Mobile Phase A to a 1000mL volumetric flask and diluting to the mark with acetonitrile.
4% Sodium Chloride: (4% NaCl) to make one (1) liter of this solution weigh 40.0g of sodium chloride (NaCl) into a 1000mL volumetric flask and dilute to the mark with laboratory de-ionized water.
Apparatus
Instrument: Finnigan TSQ with Surveyor HPLC. Liquid Chromatograph / Mass Spectrometer (LC/MS/MS) [See Instrument Parameters]
Chromatographic Column: Phenomenex LUNA 5µm C18 150 x 2mm
Food Processor: Robot-Coupe model R10, or equivalent.
Centrifuge: Must be capable of holding 50mL centrifuge tubes and 3000 revolutions per minute (rpm).
Equipment: Wrist-Action Mechanical Shaker, Vortex Mixer, and Nitrogen Evaporator with heated water bath.
Aspiration Device: Fit a bored stopper in a trap flask. Connect arm of flask to vacuum source with vacuum hosing. Snugly insert a length of Teflon or flexible plastic tubing into the bore hole of the stopper. Attach a disposable pipettor tip to the "working end" of the tubing. This end is the snout that is used to suction off the hexane from the aqueous layer, and the tip can be changed between each sample. This device allows for deft aspiration of the top layer of solvent if the snout tip is placed against the tube wall slightly above the liquid surface, for aspiration of the very thin solid layer that sometimes forms between aliphatic and aqueous layers.
Centrifuge Tubes: Fifty milliliter (50mL) polypropylene, conical, with screw-caps.
Syringes: 1mL polypropylene for filtering extract.
Syringe Filters: 13 mm x 0.2µm PVDF (polyvinylidene fluoride) membrane filters.
Volumetric Glassware: Various class A pipettes and flasks.
Instrument Parameters
- Chromatography
- Gradient: (see table below)
Minutes Mobile Phase A Mobile Phase B 0 100% 0% 15 20% 80% 15.5 100% 0% 20.5 100% 0% - Approximate Retention Time of CAP: ~12 minutes
- Flow Rate: 200µL/minute
- Column Oven: 40°C
- Gradient: (see table below)
- Autosampler Conditions
- Injection Volume: 10µL
- Syringe flush and wash volume: 6mL
- Sample Tray Temperature: 10°C
- Mass Spectroscopy
- Ionization: Negative Ion Electrospray
- Spray Voltage: 1.5 kV
- Sheath Gas: N2 @ 80psi
- Capillary Temperature: 350°C
- Source Offset Voltage: 5 V
- Precursor Ion (Q1): m/z 321
- Collision Gas (Q2): Argon @ 2.5 milliTorr
- Collision Voltage: 26 V
- Product Ions (Q3): m/z 257, m/z 194, m/z 176, m/z 152
- Electron Multiplier Voltage: 1.27 kV
- Ionization: Negative Ion Electrospray
Sample Preparation
One hundred grams (100g) of cooked crab meat and two hundred grams (200g) of dry ice were placed in the Robot-Coupe food processor. This mixture was then processed to a fine powder consistency. This mixture of powdered crab and dry ice was then de-gassed overnight in a freezer before proceeding. (There are a couple of safety reminders here: The mixture should not be stored in sealed containers, as the evolving gas will build up pressure presenting a possible bursting hazard. The second point is that depending on the total amount of dry ice involved, an asphyxiation hazard could develop in a walk-in freezer.) This dry-ice technique is based on the work of Bunch, et. al.(2)
Sample Extraction and Clean-up
[See Figure 1. Lab Aid, for visual flowchart of the method.] Ten grams (10g) of degassed, frozen crab powder were weighed into the first (of three) fifty milliliter (50mL) plastic centrifuge tubes. Twenty milliliters (20mL) of ethyl acetate (EtOAc) were added to the centrifuge tube and capped tightly. The tube was shaken vigorously for ten minutes, using a wrist-action mechanical shaker. The tube was then centrifuged for five minutes at 3000 RPM, and the supernatant was decanted into the second centrifuge tube. Another 20mL portion of EtOAc was added to the sample; the tube was capped and vigorously shaken for about 5 minutes. The sample tube was centrifuged again for five minutes at 3000 RPM and the supernatant was also decanted into the second centrifuge tube. The first sample tube with the crab pellet was then discarded (it is advisable to allow the tube to dry in a fume hood before placing in the trash).
The extract in the second sample tube was reduced to dryness with a stream of nitrogen in a water bath at 45-50°C (or until all the EtOAc is gone and just a drop of non-evaporating liquid remains). At this time two milliliters of methanol was added to the second sample tube, capped and spun on the vortex mixer for about a 30 seconds. Twenty five milliliters (25mL) of 4% NaCl solution and twenty milliliters (20mL) of heptane were added to the second sample tube. This mixture was then vigorously shaken (by hand) for about thirty seconds, and then allowed to separate for several minutes (or until any emulsion breaks up). The top layer of heptane was removed by aspiration and discarded. The de-fatting extraction was then repeated with another 20mL aliquot of heptane, and this too was removed and discarded.
The chloramphenicol was then extracted from the aqueous phase remaining in the second centrifuge tube, by adding fifteen milliliters (15mL) of EtOAc, capping tightly and shaking vigorously by hand for about one (1) minute. The mixture was then allowed to stand for several minutes, or until the upper organic phase was clear. It is important that all emulsion be broken before the organic phase is removed. It sometimes proves necessary to centrifuge the tube to break up an especially stable emulsion. The organic phase is transferred to the third centrifuge tube and the extraction repeated with a second 15mL aliquot of EtOAc. (If an emulsion is particularly stubborn, even after centrifugation, transfer what volume you can without contamination during the first transfer step, and then again as much as you can with the second transfer step. Usually by the second extraction, the emulsion behaves much better.) After both aliquots had been transferred to the third tube, the extract was again reduced to dryness with a stream of nitrogen in a water bath at 45-50°C.
The dry residue was re-dissolved in 1.00 mL of Diluent, swirled and briefly vortexed, and then transferred to a 1mL polypropylene syringe and filtered through a 0.2µm membrane filter, into a auto-sampler vial. The extract was then ready for analysis.
Standards
The primary stock standard was made by accurately weighing about 20.0 mg of USP Reference Standard Chloramphenicol and then diluting to 50.0 mL in methanol. This gives a standard of about 400,000 ng/mL.
Working Standard 1 (WS1) was made by pipetting 1.00 mL of primary stock standard into a 100 mL volumetric flask and diluting to the mark with Diluent, giving a standard of about 4000 ng/mL. Working Standard 2 (WS2) was made by pipetting 1.00 mL of WS1 into a 100 mL volumetric flask and diluting to the mark with Diluent, giving a standard of about 40 ng/mL.
The linearity/calibration standards were made according to the Table 1, by taking aliquots of WS2 and diluting to 10.0 mL, and then taking equal portions (200 uL each) of the diluted standard and blank crab extract, mixing together, to give the final "tissue equivalent" standard. Blank crab extract consists of the final extract of unfortified crab, taken through the extraction method. To have enough of this blank crab extract on hand, one can elect to: extract multiple crab blanks; perform a scaled-up extraction of a larger aliquot of crab tissue; or create a reserve pool of previously extracted crab blanks. Blank crab extract is added to the plain standard CAP to help equalize any possible matrix effects in the chromatography or fragmentation between samples and standards. Although only four standards were made here (same as in LIB 4290), we recommend regulatory runs to use five levels of standard, and to have the lowest standard = 50% of the spike or target level.
| STD Name | Aliquot of WS2 | Final Volume | Standard Concentration | Standard Concentration After 1:1 Dilution with crab extract | Equivalent ppb in extracted Tissue (10 g) |
|---|---|---|---|---|---|
| A | 0.5 mL | 10.0 mL | 2.0 ng/mL | 1.0 ng/mL | 0.1 ppb |
| B | 1.0 mL | 10.0 mL | 4.0 ng/mL | 2.0 ng/mL | 0.2 ppb |
| C | 2.5 mL | 10.0 mL | 10.0 ng/mL | 5.0 ng/mL | 0.5 ppb |
| D | 5.0 mL | 10.0 mL | 20.0 ng/mL | 10.0 ng/mL | 1.0 ppb |
Sample Spiking and Method Design
Crab for analysis was retail frozen cooked crab meat imported from Chile. Individual 10 g samples were fortified to contain 0.10, 0.25, 0.50, and 1.0 ng CAP per g of crab, using 10, 25, 50 and 100 uL of Spiking Soln. Spiking Soln was prepared at a concentration of 100 ng/mL, by diluting an appropriate aliquot of WS1 up to 25.0 mL using 1:1 methanol:water. Five replicates of each of the four fortification levels were analyzed; however, two sets, for a total of ten replicates, of the 0.10 ng/g concentration were analyzed. A minimum of five control (or "blank") replicates were also analyzed. This provided a population of n=30.
Since multiple samples were being handled concurrently, sample extraction generally took about a day and a half to perform (not including sample preparation). Partially extracted samples were held in a refrigerator overnight between extraction steps; and finished extracts can also be held in a refrigerator prior to analysis.
For each day of instrumental analysis, the linearity standards were injected prior to the samples, between sample sets, and at the end of all the samples (total of three times). Injections of Diluent were made between standard and sample sets, as well as for the first run of the day. Injections of crab control samples were made before the first set of samples. For regulatory purposes, we also advise making five replicate injections for system suitability purposes of a single standard prior to the first set of calibration standards, and injecting a reagent blank after the first set of calibration standards (and diluent run). It would also be sufficient to only inject the middle standard (which would have a concentration at or near the target level)--instead of the whole set of standards comprising the curve--between sample sets and at the end.
For LC/MS/MS analysis, four daughter ions (m/z 152, 176, 194 and 257) were monitored off of the m/z 321 precursor ion. Determination was based on the standard curve of the peak areas of the m/z 152 daughter ion (the base peak), for standard solutions equivalent to 0.10, 0.20, 0.50, and 1.0 ppb in tissue (made with control crab extract). Since three sets of standards were run each day, three sets of data points were used for each of the standard concentrations that made up the curve (=12 data sets per curve).
Results and Discussion
Quantitation. Average absolute recoveries of the five fortification sets ranged from 51-86% [Table 2], and none of five replicates of each fortification level varied between themselves more than 1 percentage point, showing excellent daily repeatability (intra-assay %RSD = 0.74). Absolute recoveries of the 1.0 and 0.50 ppb fortification levels were within 2 percentage points of each other (86% and 84%, respectively); however, recovery noticeably dropped off below 0.5 ppb. The correlation coefficients (r) of the standard curves were greater than 0.999. For daily use in a regulatory lab, the limit of quantitation (LOQ) is routinely taken as equivalent to the lowest standard, anything less being reported as a "trace." However, the recoveries of the 0.10 ng/g fortified crab placed their responses below the lowest standard. For research purposes, we extrapolated the curve and reported recovered concentrations in Table 1, because the signals were well over the common 3 × signal-to-noise ratio for Limit of Detection. Note: using essentially the same method for CAP in shrimp and crayfish, we found that crab gave lower recoveries, and also tended to give more emulsions.
Confirmation. For identification purposes the retention times of CAP in the standards and the samples were compared and typically agreed within 0.1 minute. But more importantly, the ion ratios (of each daughter ion versus the base daughter ion) of the fortified crab versus those of the chloramphenicol standards, agreed within 10% (relative) at chloramphenicol concentrations of 0.25-1.0 ppb. However, the ratios increased to up to 21% for the m/z 257 ion in the 0.10 ppb (2) fortified crab [Table 3]. The %RSD of the ion ratios of the standards (across all concentration levels) were 4.1-8.8%. Crab at a given fortification concentration was compared against the standard at the same concentration (or rather, the average of the ion ratio of each ion of the three standard injections).
The criteria for successful confirmation of the presence of CAP in crab meat can be summarized:
- A retention time agreement between sample and (matrix) standard of within ±0.3 minutes.
- Parent ion of m/z 321.
- Daughter ions of m/z 152, 176, 194 and 257; no other significant peaks present.
- Daughter ion m/z 152 is the base peak.
- When comparing the numbers for a sample versus a standard, the ratios of m/z 176, 194, and 257 versus m/z 152 are within 10% (relative).
- The signal to noise ratio for the weakest daughter ion (m/z 176) should be > 5 X.
Conclusions
This method is straightforward and uses basic wet chemistry techniques--which allows it to be used successfully by a wide range of analysts. It minimizes use of solvents and glassware, and we have found it to be useful in a high-throughput environment (however, it still takes around one to one and a half days to perform the extraction on multiple concurrent subs). It simultaneously provides determination and confirmation of chloramphenicol in cooked crab meat, which is useful in a regulatory situation. The method is also applicable to shrimp and crayfish.
Acknowledgements
Many thanks to PRLNW Chemists: Rachel Tomlin for her friendly and efficient sample preparation; to Jan Bussey for her kind assistance with the LC/MS/MS; and especially to Barbara Neuhaus for her technical assistance and trailblazing of methodology.
References
- "LC/MS/MS Analysis of Chloramphenicol in Shrimp," Barbara K. Neuhaus, Jeffrey A. Hurlbut, and Walter Hammack. (2002) Laboratory Information Bulletin No. 4290.
- "Homogeneous Sample Preparation of Raw Shrimp with the Aid of Dry Ice," Elaine Bunch, Diane Altwein, Lloyd Johnson, Joyce Farley, and Amy Hammersmith. (1995) J. AOAC Int, 78, 883-887.
- Allen Pfenning, Jose Roybal, Heidi Rupp, Sherri Turnipseed, Steven Gonzales, Jeffrey Hurlbut. (2000) J.AOAC Int., 83, 26ff.
- Allen Pfenning, Mark Madson, Jose Roybal, Sherri Turnipseed, Steven Gozales, Jeffrey Hurlbut, and G. Salmon. (1998) J.AOAC Int., 81, 714ff.
- "Confirmation of Chloramphenicol Residue in Crab by Electrospray LC/MS," Allen Pfenning, Sherri Turnipseed, Jose Roybal, Mark Madson, Rebecca Lee, and Joseph Storey. (2002) Laboratory Information Bulletin No. 4294.
| Fortification Level n=5 | 0.10 ppb (1) | 0.10 ppb (2)* | 0.25 ppb | 0.50 ppb | 1.0 ppb | |
|---|---|---|---|---|---|---|
| Ave. found, ppb | 0.053 | 0.051 | 0.17 | 0.42 | 0.86 | |
| Ave. recovery, % | 53 | 51 | 67 | 84 | 86 | Intra-assay average |
| RSD, % | 0.75 | 0.91 | 0.67 | 0.54 | 0.81 | 0.74 |
*Repeated extraction and analysis because original value seemed low; however, it seems to be the trend for low concentrations.
| % ion ratio vs. m/z 152 | ||||
|---|---|---|---|---|
| Fortification Level* | m/z 257 ion | m/z 194 | m/z 176 | |
| 1.0 ppb | Ave. of Standards | 57 | 45 | 23 |
| Ave. of Samples | 55 | 45 | 23 | |
| Relative % Difference of Sample vs. Std. | 2.4 | 0.7 | 0.9 | |
| 0.5 ppb | Ave. of Standards | 57 | 45 | 25 |
| Ave. of Samples | 55 | 46 | 23 | |
| Relative % Difference of Sample vs. Std. | 3.1 | 2.5 | 6.8 | |
| 0.25 ppb | Ave. of Standards | 56 | 45 | 24 |
| Ave. of Samples | 60 | 48 | 23 | |
| Relative % Difference of Sample vs. Std. | 7.2 | 7.7 | 5.2 | |
| 0.10 ppb (1) | Ave. of Standards | 51 | 45 | 23 |
| Ave. of Samples | 61 | 52 | 26 | |
| Relative % Difference of Sample vs. Std. | 21.1 | 16.0 | 10.3 | |
| 0.10 ppb (2) | Ave. of Standards | 50 | 49 | 25 |
| Ave. of Samples | 55 | 44 | 23 | |
| Relative % Difference of Sample vs. Std. | 10.8 | 9.3 | 5.3 | |
*n=5 for the samples, n=3 for the standards
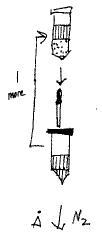
Figure 1. Lab Aid
10 g sample
+
ADD 20 mL EtOAc
shake 10 min on wrist-action mechanical shaker
centrifuge 5 min @ 3000 rpm
decant into fresh tube
Repeat Extraction & Centrifugation
dry under N2 @ 45-50°C to dryness
ADD 2 mL methanol
+
ADD 4% NaCl soln up to 25 mL mark [soln: 12 g NaCl / 300 mL H20]
briefly shake or vortex (~10 s)
ADD 20 mL heptane
briefly shake or vortex (~30 s); centrifuge if emulsion for 5 min @ 3000 rpm
aspirate off heptane (discard heptane)
Repeat Heptane Extraction
ADD 15 mL EtOAc
briefly shake or vortex (~30 s)
centrifuge if emulsion for 5 min @ 3000 rpm
transfer EtOAc to fresh tube
Repeat EtOAc Extraction & Transfer
dry under N2 at 45-50°C
wash down sides with ~2 mL EtOAc
take to dryness
ADD 1.00 mL of diluent [50% methanol] and vortex (~30 s)
syringe filter using 0.2 um PVDF into HPLC tube and cap



![ADD 2 mL methanol + ADD 4% NaCl soln up to 25 mL mark [soln: 12 g NaCl / 300 mL H20] ADD 2 mL methanol + ADD 4% NaCl soln up to 25 mL mark [soln: 12 g NaCl / 300 mL H20]](/files/repeat_heptane_extraction.gif)
![ADD 1.00 mL of diluent [50% methanol] and vortex (~30 s) ADD 1.00 mL of diluent [50% methanol] and vortex (~30 s)](/files/add_1ml_of_diluent.gif)