FDA Warns about the risks associated with the investigational use of Venclexta in Multiple Myeloma
[3/21/2019] The U.S. Food and Drug Administration is alerting health care professionals, oncology clinical investigators and patients about the risks associated with the investigational use of Venclexta (venetoclax) for the treatment of patients with multiple myeloma based on data from a clinical trial. Venclexta is not approved for the treatment of multiple myeloma.
FDA reviewed data from the BELLINI clinical trial (NCT02755597, Study M14-031) evaluating the use of Venclexta combined with bortezomib, a proteasome inhibitor, and dexamethasone in patients with multiple myeloma. The interim trial results demonstrated an increased risk of death for patients receiving Venclexta as compared to the control group. On March 6, 2019, the FDA required no new patients be enrolled on the Bellini trial. Patients who are receiving clinical benefit can continue treatment in the trial after they reconsent. More information about the BELLINI clinical trial findings can be found below (see statistical analysis section below).
This statement does not apply to patients taking Venclexta for an approved indication. Patients taking Venclexta for an approved indication should continue to take their medication as directed by their health care professional. Venclexta is safe and effective for its approved uses.
The FDA suspended enrollment in other ongoing multiple myeloma clinical trials of Venclexta. Patients who are receiving clinical benefit can continue treatment in these trials after they reconsent. FDA will be working directly with sponsors of Venclexta, as well as other investigators conducting clinical trials in patients with multiple myeloma, to determine the extent of the safety issue. The agency will communicate any new information as appropriate.
Health care professionals and patients are encouraged to report any adverse events or side effects related to the use of these products and any drugs to FDA’s MedWatch Adverse Event Reporting program by:
- Completing and submitting the report online at MedWatch online voluntary reporting form; or
- Downloading and completing the form, then submitting it via fax at 1-800-FDA-0178.
Statistical Analysis and Findings
Following is a summary of findings from the BELLINI clinical trial. The FDA conducted a safety and effectiveness analysis based on a November 26, 2018 data cutoff date for the BELLINI Study.
BELLINI
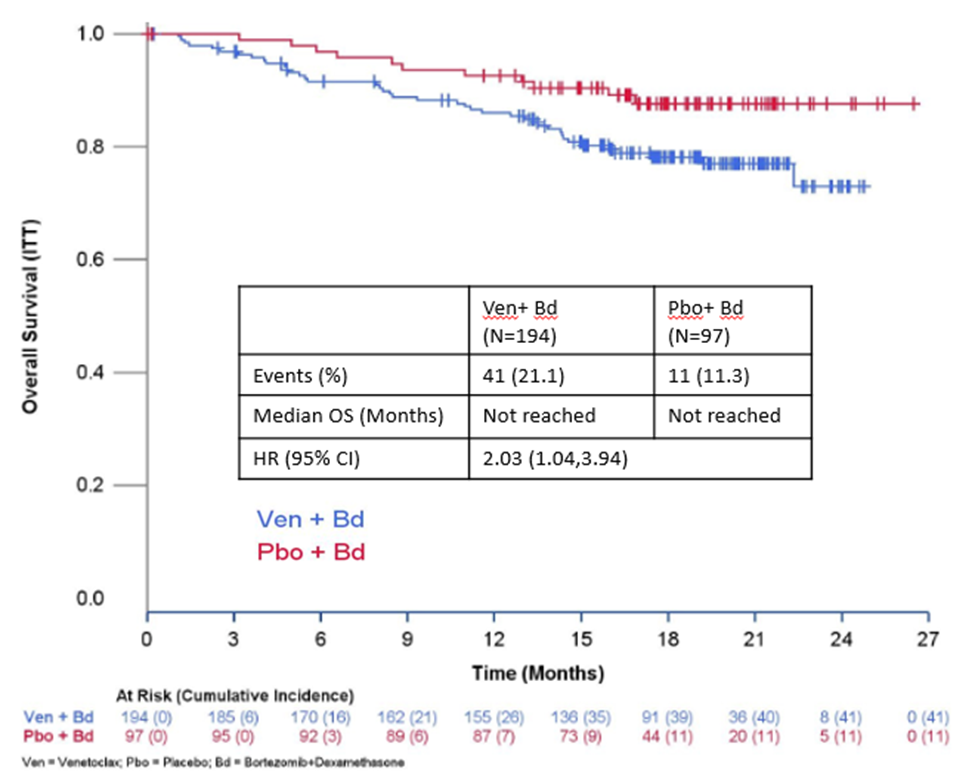
Bellini is a phase 3, double-blind, randomized, controlled trial of bortezomib and low-dose dexamethasone with or without venetoclax in patients with relapsed and refractory multiple myeloma who have received 1 to 3 prior lines of therapy.
Using a data cutoff date of November 26, 2018, an evaluation of safety and efficacy was performed. There were 291 randomized patients included in the analysis. The median follow-up was approximately 17.9 months. At the interim analysis for overall survival, there were 41/194 (21.1%) deaths on the venetoclax-containing investigational arm and 11/97 (11.3%) deaths on the placebo arm. The hazard ratio (HR) of the venetoclax-containing investigational arm compared to the placebo arm was 2.03 (95% CI: 1.04,3.94), increasing the relative risk of death by approximately two-fold compared to the placebo arm.
Source: FDA analysis
The median PFS (95% CI) was 22.4 months (15.3, -) for the venetoclax arm and 11.5 months (9.6, 15.0) for the placebo arm. The estimated HR was 0.63 (0.44, 0.90). The objective response rate (ORR) was 82.0% (75.8, 87.1) in the investigational arm compared to 68.0% (57.8, 77.1) in the placebo arm. MRD negativity rate (10-5) was 13.4% (8.9, 19.0) compared to 1.0% (0.0, 5.6) in the placebo arm.
The incidence of severe, grade 3-5 toxicity (86.5% vs. 87.5%, venetoclax vs. placebo arm) and serious adverse events (48.2% vs.50.0%) was similar between the two arms. The incidence of infections (by System Organ Class) was 79.8% in the venetoclax arm and 77.1% in the placebo arm. The incidence of pneumonia was 20.7% in the venetoclax arm and 15.6% in the placebo arm. Serious infection related adverse events were reported in 28.0% of patients in the venetoclax arm and 27.1% in the placebo arm. Serious adverse events of pneumonia were reported in 14.0% of patients in the venetoclax arm and 12.5% of patients in the placebo arm. Common non-disease progression causes of death identified in the venetoclax arm were: sepsis, pneumonia, and cardiac arrest.