FDA Drug Safety Communication: Important change to heparin container labels to clearly state the total drug strength
Safety Announcement
Additional Information for Patients and Caregivers
Additional Information for Health Care Professionals, Hospitals, and Pharmacies
Additional Background Information
Safety Announcement
[12-06-2012] The U.S. Food and Drug Administration (FDA) is notifying health care professionals, caregivers, and patients about a change to the container and carton labels for heparin products, which are blood-thinning agents that prevent the formation of blood clots.
|
Facts about heparin |
|
This label change will require manufacturers of Heparin Lock Flush Solution, USP and Heparin Sodium Injection, USP to clearly state the strength of the entire container of the medication followed by how much of the medication is in 1 milliliter (mL). These modifications will eliminate the need for health care professionals to calculate the total amount of heparin medication in a product containing more than 1 mL, thereby reducing the risk of miscalculations that may result in medication errors.
FDA supports the United States Pharmacopeia (USP)* proposal to revise the labeling section of USP monographs for Heparin Lock Flush Solution, USP and Heparin Sodium Injection, USP to clearly state the total drug strength on the label. This will ensure that labels for heparin products comply with USP’s general requirements for all small-volume injectable products, which currently display the total drug content.
Health care professionals, caregivers, and patients should be aware that that there will be a transition period before and after the official implementation date on May 1, 2013, during which both the current heparin container labels and the revised heparin container labels will be available in the marketplace. To minimize the potential for medication errors, users should consider separating the supplies of “current” and “revised” labeled heparin, and use all of the supplies of the “current” heparin before using products with the “revised” container label.
*USP is a scientific nonprofit organization that develops standards for the identity, strength, quality, and purity of drugs and drug ingredients marketed in the U.S. These standards are published in USP’s official compendia, U.S. Pharmacopeia and National Formulary.
Current and Revised Heparin Labels
|
Current Heparin Label |
Revised Heparin Label |
The proposed revision to the labeling sections in the heparin monographs will require the labels to comply with the USP standards for injectable medications, specifically USP 35 -NF 30 General Chapter <1> Injections section.
The following formats are those FDA considers acceptable for heparin vials and syringes that contain more than 1 mL:
The strength per total volume should be the primary and prominent expression on the principal display panel of the label, followed in close proximity by strength per mL enclosed by parentheses.
Example 1:
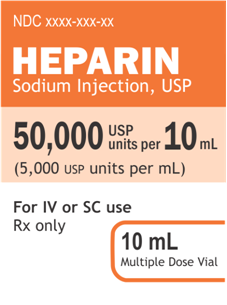
50,000 USP units per 10 mL
(5,000 USP units per mL)Example 2:
50,000 USP units/10 mL
(5,000 USP units/mL)
The following format is acceptable for contents of less than 1 mL:
The strength per fraction of a mL should be the only expression of strength.
100 USP units/0.5 mLStrength per single mL should be expressed as mg/mL, not mg/1 mL.
5,000 USP units/mL
Additional Information for Patients and Caregivers
- Always ask your health care professional to look at the label on the heparin container and to check the dose and volume to be administered.
- Contact your health care professional if you have any questions or concerns about heparin.
- Report medication errors or side effects from the use of heparin to FDA’s MedWatch program, using the information in the "Contact FDA" box at the bottom of this page.
Additional Information for Health Care Professionals, Hospitals, and Pharmacies
- To minimize the potential for medication errors, hospitals and pharmacies may wish to consider separating the supplies of “current” and “revised” labeled heparin and exhausting the supplies of the “current” heparin before transitioning to products with the “revised” label.
- Always look at the label on the heparin vial being dispensed and counsel the patient or caregiver on how to administer the correct dose.
- Report medication errors or adverse events involving heparin to the FDA MedWatch program, using the information in the "Contact FDA" box at the bottom of this page.
Additional Background Information
In 2003, the United States Pharmacopeia’s (USP) Safe Medication Use Expert Committee became aware of medication errors involving the expression of strength in the labeling for all injectable products. Containers labeled with the strength per mL were often misunderstood as the total drug content, which could result in dosing errors with serious consequences to patients. To address this safety issue, the USP Parenteral Products―Industrial Expert Committee approved the new “Strength and Total Volume for Single- and Multiple-Dose Injectable Drug Products” section of the USP General Chapter <1> Injections in 2007, and the text became official on February 1, 2009. General Chapter <1> requires that the strength per total volume should be the primary and prominent expression of strength on the principal display panel of the label, followed in close proximity by strength per mL enclosed by parentheses. Container labels that have already been changed to state the strength per total volume have had no reported medication errors.
Since 2009, concerns have arisen about the conflict in labeling requirements between the Heparin Sodium Injection and Heparin Lock Flush Solution monographs and the General Chapter <1> Injections section on “Strength and Total Volume for Single- and Multiple-Dose Injectable Drug Products.” The labeling requirement in the current heparin monographs states that the label must reflect only strength per mL, except it also allows for single-dose vials to be labeled additionally to indicate the total drug content. To address this conflict, USP has proposed revising the labeling section of the heparin monographs to ensure that the heparin container labels comply with the USP General Chapter <1> Injections section.
The proposed revision to the labeling sections in the heparin monographs will require the container labels to comply with the USP 35 -NF 30 General Chapter <1> Injections section that reads in part:
“[T]he strength per total volume should be the primary and prominent expression on the principal display panel of the label, followed in close proximity by strength per mL enclosed by parentheses. For containers holding a volume of less than 1 mL, the strength per fraction of a mL should be the only expression of strength. Strength per single mL should be expressed as mg/mL, not mg/1 mL.”
The official implementation date for the USP Heparin Lock Flush Solution and USP Heparin Sodium Injection monographs is May 1, 2013. Manufacturers are expected to have revised their heparin labels accordingly by that time. A transition period will occur during which both the current heparin container labels and the revised heparin container labels will appear in the marketplace.
Related Information
- Information on Heparin
- FDA Drug Safety Podcast: Important change to heparin container labels to clearly state the total drug strength
- Comunicado de la FDA sobre la seguridad de los medicamentos: Cambio importante en la etiqueta del envase de heparina para indicar claramente la potencia total del medicamento