FDA Drug Safety Communication: FDA reporting permanent skin color changes associated with use of Daytrana patch (methylphenidate transdermal system) for treating ADHD
[ 06-24-2015 ]
The U.S. Food and Drug Administration (FDA) is warning that permanent loss of skin color may occur with use of the Daytrana patch (methylphenidate transdermal system) for Attention Deficit Hyperactivity Disorder (ADHD). FDA added a new warning to the drug label to describe this skin condition, which is known as chemical leukoderma.
Patients or their caregivers should watch for new areas of lighter skin, especially under the drug patch, and immediately report these changes to their health care professionals. Patients should not stop using the Daytrana patch without first talking to their health care professionals. We are recommending that health care professionals consider alternative treatments for patients who experience these skin color changes.
The Daytrana patch treats ADHD by working to increase attention and decrease restlessness in children and adolescents who are overactive, cannot concentrate for very long, or are easily distracted and impulsive.
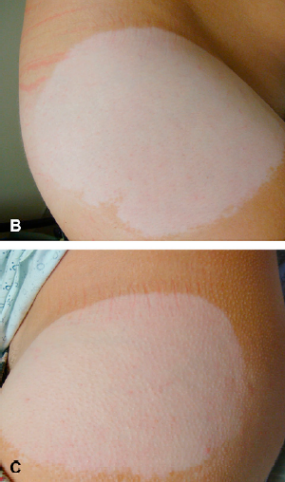
Chemical leukoderma is a skin condition that causes the skin to lose color due to repeated exposure to specific chemical compounds. The condition is not physically harmful, but it is disfiguring. The areas of skin color loss described with the Daytrana patch ranged up to 8 inches in diameter. This condition is not thought to be reversible, which may cause emotional distress (See Photos).
We reviewed cases of chemical leukoderma associated with the Daytrana patch reported to the FDA Adverse Event Reporting System (FAERS) database and described in the medical literature. FAERS includes only reports submitted to FDA so there are likely additional cases about which we are unaware. FDA identified 51 FAERS cases from April 2006 to December 2014 and one published case that was not recorded in FAERS. The time to onset of leukoderma after starting Daytrana ranged from 2 months to 4 years. All of the patients described a decrease in or loss of skin color. In most cases, the loss of skin color was limited to the areas around where the patch was rotated. However, a small number of patients also reported skin color changes on parts of the body where the patch was never applied. In all cases, the decreased skin color was permanent.
We urge patients and health care professionals to report side effects involving the Daytrana patch to the FDA MedWatch program, using the information in the “Contact FDA” box at the bottom of the page.
- The Daytrana patch (methylphenidate transdermal system) is a prescription patch used to treat ADHD. It belongs to the group of medications called central nervous system stimulants. It must be applied to the skin once daily on the hip and worn for 9 hours.
- The Daytrana patch works by increasing attention and decreasing restlessness in children and adolescents who are overactive, cannot concentrate for very long, or are easily distracted and impulsive. This medication is used as part of a total treatment program that includes social, educational, and psychological treatment.
- The Daytrana patch is applied to the hip area and delivers medication through the skin into the bloodstream. It is available in four different dosage strengths. The patch formulation does not require swallowing a pill, which is beneficial for young children.
- The Daytrana patch application site should be rotated daily, placing it on a different spot and on the opposite hip, if possible.
- Common side effects reported with the Daytrana patch and other stimulant medications include increased blood pressure and heart rate, nausea, decreased appetite and weight, aggressive behavior, anger, and irritability.
- In 2014, approximately 109,000 patients received prescriptions for Daytrana dispensed from U.S. outpatient retail pharmacies, which was a decrease from 136,500 patients in 2010.1
- Permanent loss of skin color may occur with use of the Daytrana patch (methylphenidate transdermal system) for ADHD.
- Patients or their caregivers should monitor for signs of lighter areas of skin, especially around the areas where the drug patch has been placed, and report any changes in skin color to their health care professionals.
- Patches of lighter skin may appear only in the areas where the patch has been applied, or it may appear on the body in places where the patch was never applied (See Photos).
- This skin condition, called chemical leukoderma, is not physically harmful, but it is disfiguring and not thought to be reversible so can cause emotional distress.
- Daytrana is a long-acting methylphenidate formulation that does not require the patient to swallow a pill.
- Do not stop using the Daytrana patch without first discussing it with your health care professional.
- Discuss any questions or concerns about the Daytrana patch with your health care professional.
- Carefully read the patient Medication Guide that comes with the filled prescription.
- Report any side effects from the Daytrana patch to your health care professional and the FDA MedWatch program, using the information in the Contact FDA box at the bottom of the page.
- Postmarketing reports of acquired skin depigmentation or hypopigmentation of the skin, consistent with chemical leukoderma, have been associated with the use of the Daytrana patch.
- Discontinue the Daytrana patch if loss of pigmentation occurs and consider other long-acting treatment options for ADHD.
- Advise patients to seek medical attention immediately if they notice signs of skin depigmentation or hypopigmentation while on treatment.
- Encourage patients and their caregivers to read the patient Medication Guide provided with their Daytrana patch prescriptions.
- Report adverse events involving the Daytrana patch to the FDA MedWatch program, using the information in the "Contact FDA" box at the bottom of this page.
FDA reviewed 51 cases reported to the FDA Adverse Event Reporting System (FAERS) database from April 2006 through December 2014, and one case published in the medical literature2 of chemical leukoderma in association with the Daytrana patch. One-third of the cases reported a diagnosis of vitiligo; however, all of the cases described a decrease or loss of pigmentation at the application site that is consistent with the diagnostic criteria of chemical leukoderma.
Of the 51 FAERS cases reviewed, 43 cases reported that the leukoderma localized to the areas where the patch was applied, and seven reported depigmentation of skin at the application site in addition to other areas of the body. The remaining case did not provide enough information regarding the affected site to determine the characteristics of leukoderma.
The time to onset of leukoderma after starting Daytrana ranged from 2 months to 4 years. Thirteen cases reported medications prescribed to reverse the loss of skin color, with a possible slight improvement reported in three of those. None of the cases reported a resolution after discontinuing Daytrana. Three cases reported either a local or a distal leukoderma even after Daytrana was discontinued.
- Source: IMS Health Total Patient Tracker (TPT), 2010-2014, Extracted MAY2015.
- Ghasri P, Gattu S, Saedi N, Ganesan AK. Chemical leukoderma after the application of a transdermal methylphenidate patch. J Am Acad Dermatol. 2012 Jun;66(6):e237-8.
Higher magnification of the depigmented patches on the (B) right and (C) left hips.
Copyright permission from American Academy of Dermatology (Vol. 66, Iss 6, June 2012)
Drug Safety Communication (PDF - 85KB)