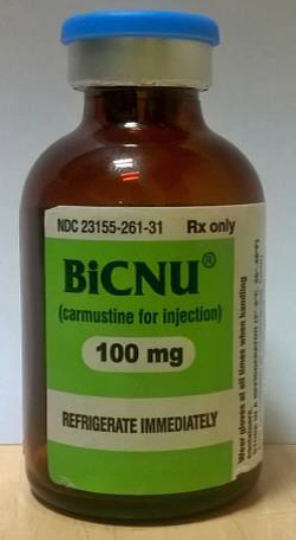
FDA advises health care professionals that counterfeit BiCNU has been discovered in some foreign countries
Comparison of the authentic BiCNU product vial to the counterfeit product vial
| Authentic BiCNU Vial | Counterfeit Product Vial |
|---|---|
- The authentic product has a blue flip top; the counterfeit product has a gray flip top
- The NDC number on the authentic product vial should end with -31, not -41.
[05/12/2016] The FDA is informing health care professionals that a counterfeit version of the FDA approved cancer drug, BiCNU (carmustine for injection) 100 mg, has been detected in some foreign countries. There is no indication at this time that counterfeit BiCNU has entered the legitimate U.S. drug supply chain and no indication that any U.S. patients have received counterfeit BiCNU.
The authentic product is approved to treat different types of brain cancer, multiple myeloma, and lymphoma (Hodgkin’s and non-Hodgkin’s). BiCNU is manufactured by Emcure Pharmaceuticals Ltd. and distributed in the United States by Heritage Pharmaceuticals Inc.
The FDA is advising health care professionals to carefully inspect the BiCNU vial as an added precaution to ensure the product administered to patients is authentic.
BiCNU is available as a vial of BiCNU and dehydrated alcohol co-packaged together. While the NDC on the outer package of the authentic and counterfeit versions might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging.
The product may also be counterfeit if the vial displays the following lot numbers, batch numbers, manufacturing dates, and expiration dates. Following is identifying information for the counterfeit lots that have been reported to the FDA to date:
|
Product |
Exp. |
Mfg. |
Lot |
Batch No. |
|
BiCNU |
01/18 |
02/16 |
BCEM771322 |
EM/BC20161990 |
|
Diluent |
01/18 |
02/16 |
SBCDA224736 |
EM/BCD2220 |
|
BiCNU |
12/17 |
01/16 |
BCEM771318 |
EM/BC20151896 |
|
Diluent |
12/17 |
01/16 |
SBCDA224732 |
EM/BCD2216 |
|
BiCNU |
10/17 |
11/15 |
BCEM771317 |
EM/BC20151895 |
|
Diluent |
10/17 |
11/15 |
SBCDA224731 |
EM/BCD2215 |
The FDA urges health care professionals to purchase drug products only from legitimate suppliers. Health care professionals are encouraged to report sales solicitation of suspect drug products by:
- Calling the FDA’s Office of Criminal Investigations (OCI) at 800-551-3989;
- Reporting to OCI; or
- Emailing DrugSupplyChainIntegrity@fda.hhs.gov.
Health care professionals and patients should report adverse events related to the use of any suspect medications to the FDA’s MedWatch Adverse Event Reporting program by:
-
Completing and submitting the report online at www.fda.gov/medwatch/report.htm; or
-
Downloading and completing the form, then submit it via fax at 1-800-FDA-0178.
The FDA is committed to protecting public health by securing the drug supply chain against counterfeit and unapproved medications that enter the United States through fraudulent sources. Visit Know Your Source: Protecting Patients from Unsafe Drugs for information about how to safely purchase prescription drugs for your patients.