Drug Trials Snapshots: OXBRYTA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the OXBRYTA Package Insert for complete information.
OXBRYTA (voxelotor)
ox brye ta
Global Blood Therapeutics
Approval date: November 25, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OXBRYTA is used for the treatment of sickle cell disease (SCD) in patients 12 years of age and older.
SCD is an inherited blood disorder in which the red blood cells are abnormally shaped (in a crescent or "sickle" shape) because of an abnormal type of hemoglobin (the part of the red blood cell that carries oxygen to all parts of the body). The disease is characterized by hemolytic anemia and recurrent painful vaso-occlusive crises (VOC).
How is this drug used?
Three OXBRYTA tablets (total of 1500 mg) are taken once daily by mouth.
What are the benefits of this drug?
Fifty one percent (46/90) of patients treated with OXBRYTA achieved an increase in hemoglobin level of at least 1g/dl in comparison to 6.5% (6/92) of patients treated with placebo.
OXBRYTA was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
More trials are ongoing to assess whether there is a clinical benefit.
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy was based on Hb response rate defined as the proportion of patients who had Hb increase of > 1 g/dL from baseline to Week 24. The response rate for OXBRYTA was 51.1% (46/90) compared to 6.5% (6/92) in the placebo group (p < 0.001).
Figure 4. Patient-level Change from Baseline in Hemoglobin at Week 24 in Patients Who Completed 24 Weeks of Treatment*
*Approximately 82% of all randomized patients completed 24 weeks of treatment.
OXBRYTA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: OXBRYTA worked similarly in males and females.
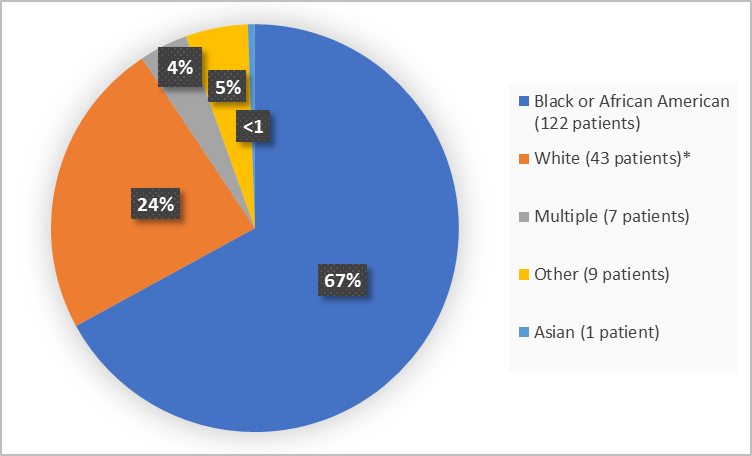
- Race: The majority of patients were Black or African Americans. The number of patients in other races were limited; therefore, differences in how OXBRYTA worked among races could not be determined.
- Age: The majority of patients were adults between 18-64 years of age. There were not enough patients older than 65 years to determine whether there was any difference in how OXBRYTA worked between older and younger patients.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
A forest plot of the subgroup analysis is presented below.
Figure 5. Primary Endpoint Analysis (Intent-to-Treat population)
HU=hydroxyurea
Adapted from FDA Review
What are the possible side effects?
OXBRYTA may cause serious allergic reactions.
The most common side effects of OXBRYTA include headache, diarrhea, abdominal pain, nausea, fatigue, rash and fever.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reaction in the Trial 1.
Table 1. Adverse Reactions (≥10%) in Patients Receiving OXBRYTA with a Difference Between Arms of >3% Compared to Placebo
|
Adverse Reaction a |
OXBRYTA |
Placebo |
|
Headache |
23 (26%) |
20 (22%) |
|
Diarrhea |
18 (20%) |
9 (10%) |
|
Abdominal Pain b |
17 (19%) |
12 (13%) |
|
Nausea |
15 (17%) |
9 (10%) |
|
Fatigue |
12 (14%) |
9 (10%) |
|
Rash c |
12 (14%) |
9 (10%) |
|
Pyrexia |
11 (12%) |
6 (7%) |
a Adverse reactions were Grades 1 or 2 except for Grade 3 diarrhea (1), nausea (1), rash (1), and rash generalized (3)
b Abdominal pain (grouped PTs) included the following PTs: abdominal pain and upper abdominal pain
c Rash (grouped PTs) includes the following PTs: rash, urticaria, generalized rash, maculo-papular rash, pruritic rash, papular rash, erythematous rash, and vesicular rash
OXBRYTA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar between males and females.
- Race: The majority of patients were Black or African Americans. The number of patients in other races were limited; therefore, differences in how OXBRYTA worked among races could not be determined.
- Age: The majority of patients were adults between 18-64 years of age. There were not enough patients older than 65 years to determine whether there was any difference in side effects between older and younger patients.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The proportion of subjects who had selected side effects by sex and age are presented below. Race subgroup differences were not analyzed because of predominance of Black or African American population.
Table 2. Subgroup Analyses of Adverse Events by Sex Group
|
|
Male |
Female |
||
|
OXBRYTA |
Placebo |
OXBRYTA |
Placebo (N = 49) n (%) |
|
|
Any TEAE |
29 (93.5) |
36 (85.7) |
54 (94.7) |
45 (91.8) (91.8) |
|
Headache |
5 (16.1) |
10 (23.8) |
18 (31.6) |
10 (20.4) |
|
Diarrhea |
5 (16.1) |
5 (11.9) |
13 (22.8) |
4 (8.2) |
Clinical Trial Data
Table 3. Subgroup Analyses of Adverse Events by Age Group
|
|
Adults |
Adolescents |
||
|
OXBRYTA |
Placebo |
OXBRYTA |
Placebo (N = 17) n (%) |
|
|
Any TEAE |
70 (94.6) |
66 (89.2) |
13 (92.9) |
15 (88.2) |
|
Headache |
21 (28.4) |
15 (20.3) |
2 (14.3) |
5 (29.4) |
|
Diarrhea |
17 (23) |
7 (9.5) |
1 (7.1) |
2 (11.8) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved OXBRYTA based on evidence from one clinical trial (Trial 1/NCT03036813) that enrolled patients with SCD disease. The trial was conducted at 60 sites in the United States, Canada, Egypt, Kenya, Lebanon, Oman, Jamaica, Great Britain, Italy, Netherlands, France and Turkey.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
Adapted from FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
* includes White, Arab, Middle Eastern
Adapted from FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age
Adapted from FDA Review
Demographics of trial participants are presented below.
Table 4. Baseline Demographics (Randomized Population)
|
Demographic Characteristic |
OXBRYTA |
Placebo |
Total |
|
Sex, n (%) |
|
|
|
|
Female |
58 (64.4) |
50 (54.3) |
108 (59.3) |
|
Male |
32 (35.6) |
42 (45.7) |
74 (40.7) |
|
Race, n (%) |
|
|
|
|
Asian |
1 (1.1) |
0 |
1 (0.5) |
|
Black or African |
59 (65.6) |
63 (68.5) |
122 (67) |
|
Multiple |
5 (5.6) |
2 (2.2) |
7 (3.8) |
|
White* |
22 (24.4) |
21 (22.8) |
43 (23.6) |
|
Other** |
3 (3.3) |
6 (6.5) |
9 (4.9) |
|
Age (Years) |
|
|
|
|
Mean (SD) |
27 (11.7) |
28 (11.5) |
27 (11.5) |
|
Median |
24 |
28 |
25 |
|
Min, Max |
12, 59 |
12, 64 |
12, 64 |
|
Age Group, n (%) |
|
|
|
|
12 -18 years |
14 (15.6) |
17 (18.5) |
31(17) |
|
≥18 years |
76 (84.4) |
75 (81.5) |
151 (83) |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
3 (3.3) |
3 (3.3) |
6 (3.3) |
|
Not Hispanic or Latino |
82(91.1) |
85 (92.4) |
167 (91.2) |
|
Not reported |
5 (5.6) |
4 (4.3) |
9 (4.9) |
|
Region, n (%) |
|
|
|
|
USA |
32 (35.6) |
34 (36.9) |
66 (36.3) |
|
Canada |
2(2.2) |
1(1.1) |
3 (1.6) |
|
Europe |
19 (21.1) |
18 (19.6) |
37 (20.3) |
|
Other |
37 (41.1) |
39 (42.4) |
76 (41.8) |
*includes White, Arab, Middle Eastern
**includes African, Dominican, Hispanic/Latino, Mixed, Missing
Adapted from FDA Review
How were the trials designed?
There was one trial that evaluated the benefit and side effects of OXBRYTA in patients with SCD. In this trial, patients were required to have a history of 1-10 VOCs in the previous 12 months and low hemoglobin levels (from 5.5 to 10.5 g/dL). Patients received at random either OXBRYTA or placebo tablets daily for 6 months. Neither the patients nor the healthcare providers knew which treatment was given until the end of the trial.
The benefit of OXBRYTA in comparison to placebo was assessed by a percentage of patients who achieved an increase in Hb level of 1g/dl over a 6 months period.
How were the trials designed?
The efficacy and safety of OXBRYTA for treatment of SCD disease were evaluated in a randomized, multicenter, placebo-controlled, double-blind trial. Patients had a history of 1-10 VOCs in the previous 12 months, and a baseline of Hb ≥ 5.5 to ≤ 10.5 g/dL . Majority had HbSS or HbS/beta0-thalassemia genotype. Patients received OXBRYTA or placebo tablets daily for 24 weeks.
Efficacy was evaluated on Hb response rate defined as the proportion of patients who had Hb increase of > 1 g/dL from baseline to Week 24.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.