Drug Trials Snapshots: INREBIC
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the INREBIC Package Insert for complete information.
INREBIC (fedratinib)
Inn-REH-bik
Celgene Corp.
Approval date: August 16, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
INREBIC is a drug used to treat adults with a rare type of bone marrow disorder called myelofibrosis.
Myelofibrosis is a chronic disorder where scar tissue forms in the bone marrow and the production of the blood cells moves from the bone marrow to the spleen and liver, causing organ enlargement.
How is this drug used?
INREBIC is a capsule taken by mouth once every day.
What are the benefits of this drug?
The benefit of INREBIC was assessed by spleen shrinkage Also, reduction of the severity of symptoms of the MF was evaluated.
Thirty-seven percent of 96 patients who received INREBIC had a decrease in the size of their spleen by 35% or more in comparison to one percent of 96 patients who received the placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below shows the efficacy analysis based on the percentage of patients with a 35% or greater reduction in spleen volume after 6 months of treatment.
Table 2. Percent of Patients Achieving 35% or Greater Reduction from Baseline in Spleen Volume at the End of Cycle 6 in the Trial
|
Spleen Response by MRI/CT at the End of Cycle 6 with a Follow-up Scan 4 Weeks Later |
INREBIC |
Placebo |
|---|---|---|
|
Number (%) of Patients with Spleen Volume Reduction by 35% or More |
35 (37) |
1 (1) |
|
p-value |
p<0.0001 |
|
Forty percent of 89 patients who received INREBIC had a decrease in myelofibrosis related symptoms by 50% or more compared to nine percent of 81 patients who received placebo as measured by the modified Myelofibrosis Symptom Assessment.
INREBIC Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: INREBIC worked similarly in men and women.
- Race: The majority of patients were White. The number of patients of other races was limited; therefore, differences in how well INREBIC worked between races could not be determined.
- Age: INREBIC worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by sex, race, and age group.
Table 3. Subgroup Analyses of Spleen Volume Reduction by Sex, Race, and Age Group
|
Subgroup |
INREBIC |
Placebo |
|---|---|---|
|
Sex |
||
|
Men |
15/54 |
1/55 |
|
Women |
20/42 |
0/41 |
|
Race* |
||
|
White |
31/86 |
1/90 |
|
Asian |
3/8 |
0/5 |
|
Age |
||
|
≤65 years |
24/61 |
0/44 |
|
>65 years |
11/35 |
1/52 |
FDA Review
What are the possible side effects?
INREBIC may cause a serious and sometimes fatal brain malfunction called Wernicke’s encephalopathy. Wernicke’s encephalopathy is a neurologic emergency and happens in patients who do not have enough vitamin B1 (thiamine).
Other serious side effects include low red blood cells (anemia), low blood platelets, vomiting, diarrhea, and abnormal liver and pancreatic tests.
The most common side effects are diarrhea, nausea, anemia, and vomiting.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes the adverse reactions that occurred in the clinical trial.
Table 4. Adverse Reactions Reported in ≥5% Patients Receiving INREBIC with a Difference between Arms of >5% during Randomized Treatment
|
Adverse Reactiona |
INREBIC |
Placebo |
||
|
All Grades |
Grade ≥3b |
All Grades |
Grade ≥3 |
|
|
Diarrhea |
66 |
5 |
16 |
0 |
|
Nausea |
62 |
0 |
15 |
0 |
|
Anemia |
40 |
30 |
14 |
7 |
|
Vomiting |
39 |
3.1 |
5 |
0 |
|
Fatigue or asthenia |
19 |
5 |
16 |
1.1 |
|
Muscle spasms |
12 |
0 |
1.1 |
0 |
|
Blood creatinine increased |
10 |
1 |
1.1 |
0 |
|
Pain in extremity |
10 |
0 |
4.2 |
0 |
|
Alanine aminotransferase Increased |
9 |
0 |
1.1 |
0 |
|
Headache |
9 |
0 |
1.1 |
0 |
|
Weight increased |
9 |
0 |
4.2 |
0 |
|
Dizziness |
8 |
0 |
3.2 |
0 |
|
Bone pain |
8 |
0 |
2.1 |
0 |
|
Urinary tract infectionc |
6 |
0 |
1.1 |
0 |
|
Dysuria |
6 |
0 |
0 |
0 |
|
Aspartate aminotransferase increased |
5 |
0 |
1.1 |
0 |
a CTCAE version 4.03
b Only 1 Grade 4 event (anemia)
c Includes cystitis.
INREBIC Prescribing Information
The table below summarizes the laboratory abnormalities during the trial.
Table 5. Selected Laboratory Abnormalities That Have Worsened from Baseline (≥20%) in Patients Receiving INREBIC with a Difference between Arms of >10% When Compared to Placebo in the Trial
|
Laboratory Parameter |
INREBIC |
Placebo |
||
|---|---|---|---|---|
|
All Grades |
Grade ≥3 |
All Grades |
Grade ≥3 |
|
|
Hematology |
||||
|
Anemia |
74 |
34 |
32 |
10 |
|
Thrombocytopenia |
47 |
12 |
26 |
10 |
|
Neutropenia |
23 |
5 |
13 |
3.3 |
|
Biochemistry |
||||
|
Creatinine increased |
59 |
3.1 |
19 |
1.1 |
|
ALT increased |
43 |
1 |
14 |
0 |
|
AST increased |
40 |
0 |
16 |
1.1 |
|
Lipase increased |
35 |
10 |
7 |
2.2 |
|
Hyponatremia |
26 |
5 |
11 |
4.3 |
|
Amylase increased |
24 |
2.1 |
5 |
0 |
INREBIC Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: Overall, the occurrence of side effects was similar between men and women with exception of more women reporting diarrhea and nausea than men.
- Race: The majority of patients were White. The number of patients of other races was limited; therefore, differences in the occurrence of side effects between races could not be determined.
- Age: The occurrence of side effects was similar between patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize most common adverse reactions by sex and age group. Subgroup analyses on race was not performed since the majority of patients were White.
Table 6. Selected Adverse Reactions by Sex Subgroups
|
Preferred Term |
INREBIC |
Placebo |
||
|---|---|---|---|---|
|
Men |
Women |
Men |
Women |
|
|
Anemia |
30 (56%) |
23 (55%) |
8 (15%) |
5 (12%) |
|
Diarrhea |
34 (63%) |
34 (81%) |
8 (15%) |
7 (17%) |
|
Nausea |
28 (52%) |
36 (86%) |
4 (7%) |
11 (27%) |
|
Thrombocytopenia |
10 (19%) |
6 (14%) |
6 (11%) |
2 (5%) |
Table 7. Selected Adverse Reactions by Age Subgroups
|
Preferred Term |
INREBIC |
Placebo |
||
|---|---|---|---|---|
|
≤ 65 Years |
>65 Years |
≤ 65 Years |
>65 Years |
|
|
Anemia |
32 (53) |
21 (60) |
5 (11%) |
8 (16%) |
|
Diarrhea |
40 (66) |
28 (80) |
6 (14%) |
9 (18%) |
|
Nausea |
38 (62) |
26 (74) |
10 (23%) |
5 (10%) |
|
Thrombocytopenia |
13 (21) |
3 (8) |
5 (11%) |
3 (6%) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved INREBIC based on evidence from one clinical trial (NCT01437787) of 192 patients with myelofibrosis. The trial was conducted in Asia, Australia, Brazil, Canada, Europe, Mexico, South Africa, and the United States.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Demographics by Sex
FDA Review
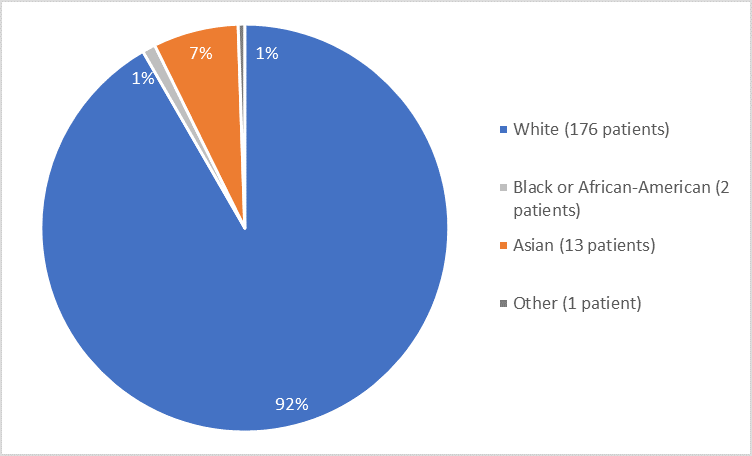
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Trial by Race
|
Race |
Number of Patients |
Percentage |
|
White |
176 |
92 |
|
Black or African American |
2 |
1 |
|
Asian |
13 |
7 |
|
Other |
1 |
Less than 1 |
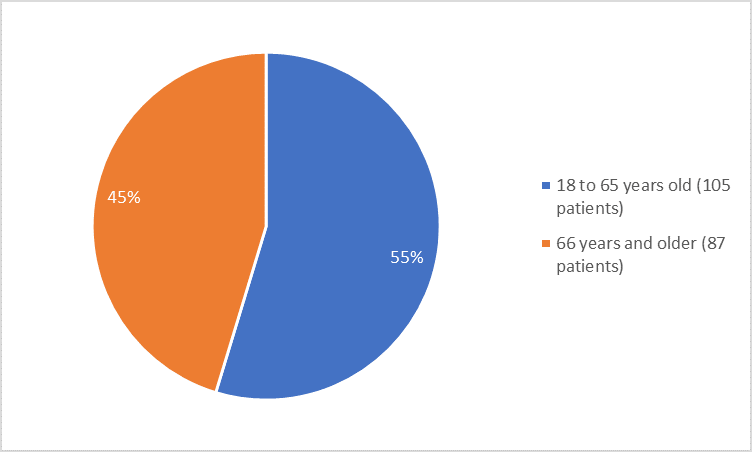
Figure 3 summarizes the percentage of patients by age group in the clinical trial.
Figure 3. Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes the demographics for the clinical trial.
Table 8. Trial Population Demographics
|
Demographic Parameters |
INREBIC |
Placebo |
|---|---|---|
|
Sex |
||
|
Men |
54 (56) |
55 (57) |
|
Women |
42 (44) |
41 (43) |
|
Race |
||
|
White |
86 (89.6) |
90 (93.8) |
|
Black or African American |
1 (1) |
1(1) |
|
Asian |
8 (8.3) |
5 (5.2) |
|
Other |
1 (1) |
0 (0) |
|
Age Group |
||
|
≤ 65 years |
61 (63.5) |
44 (45.8) |
|
> 65 years |
35 (36.5) |
52 (54.2) |
|
Ethnicity-data not collected |
||
|
Region |
||
|
United States |
9 (9) |
6 (6) |
|
Rest of the World |
87 (81) |
90 (94) |
|
Canada |
5 (5) |
3 (3) |
|
Europe |
62 (65) |
72 (75) |
|
Asia |
8 (8) |
3 (3) |
|
South America |
0 (0) |
3 (3) |
|
Australia |
12 (13) |
9 (10) |
FDA Review
How were the trials designed?
The benefit and side effects of INREBIC for myelofibrosis were evaluated in one clinical trial. The trial enrolled adult patients with myelofibrosis and enlarged spleen. All patients had the size of their spleen measured at the beginning of the trial using either MRI or CT scan. Patients received either INREBIC or placebo once each day for 6 months. Neither the patients nor the healthcare providers knew which treatment was being given during the trial. After receiving INREBIC or placebo for 6 months, patients had their spleen size measured again.
The benefit of INREBIC was evaluated by measuring the percentage of patients who achieved at least 35% shrinkage of the spleen and comparing it to the percentage of patients who received placebo. Patients also kept the diary capturing the 6 core symptoms of MF: night sweats, itching, abdominal discomfort, early satiety, pain under ribs on left side, and bone or muscle pain. The scores were evaluated at the end of the trial for assessment of symptoms improvement.
How were the trials designed?
The efficacy and safety of INREBIC were evaluated in a double-blind, randomized, placebo-controlled trial in patients with intermediate-2 or high-risk myelofibrosis, post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis with splenomegaly. Patients were randomized to receive either INREBIC or placebo once daily for at least 6 cycles. Patients underwent MRI or CT spleen volume assessment at the baseline and after the third and sixth cycle with a follow-up scan 4 weeks after Cycle 6.
The efficacy of INREBIC in the treatment of patients with primary or secondary myelofibrosis was established based upon the proportion of patients achieving greater than or equal to a 35% reduction from baseline in spleen volume at the End of Cycle 6 as measured by MRI or CT with a follow-up scan 4 weeks later.
Additional outcome included the proportion of patients with a 50% or greater reduction in Total Symptom Score from baseline to the End of Cycle 6 as measured by the modified Myelofibrosis Symptom Assessment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.