About Biomarkers and Qualification
- What is a biomarker?
- Biomarker Categories: BEST Glossary
- How can qualified biomarkers improve the drug development process?
- How are biomarkers qualified for drug development?
- Additional ways to engage with CDER
What is a biomarker?
What Are Biomarkers and Why Are They Important?
The Biomarkers, EndpointS and other Tools (BEST) glossary defines a biomarker as a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions. Molecular, histologic, radiographic, or physiologic characteristics are types of biomarkers. A biomarker is not an assessment of how an individual feels, functions, or survives.
Biomarker Categories: BEST Glossary
BEST defines seven biomarker categories: susceptibility/risk, diagnostic, monitoring, prognostic, predictive, pharmacodynamic/response, and safety.
A full biomarker description includes the biomarker name, the source/matrix, the measurable characteristic(s), and the analytic method used to measure the biomarker. A biomarker may be a single characteristic or a panel of multiple characteristics.
How can qualified biomarkers improve the drug development process?
Qualified biomarkers have the potential to provide valuable information that may reduce uncertainty in regulatory decisions during drug development.
When a biomarker is qualified, it means that it has undergone a formal regulatory process to ensure that we can rely on it to have a specific interpretation and application in medical product development and regulatory review, within the stated context of use (COU) . It is important to note that a biomarker is qualified, and not the biomarker measurement method.
How are biomarkers qualified for drug development?
The qualification process is collaborative, where the Biomarker Qualification Program works with the requestor(s) in guiding biomarker development. Multiple interested parties often work together in working groups or consortia to develop a biomarker for qualification. This approach allows for shared resources, and reduces burden on individual collaborators. In turn, this may encourage interested parties to join a DDT development effort despite limited resources.
Under the 21st Century Cures Act, biomarker qualification involves a three-stages submission process to develop a biomarker for regulatory use. For complete and quality submissions, FDA makes a decision to Accept or Not Accept . This decision is communicated to the requestor in a letter that includes feedback and recommendations for further biomarker development. During this process, there are opportunities for requestors to work collaboratively with CDER to address aspects of the biomarker’s development.
The 21st Century Cures Act also includes transparency provisions requiring, among other things, that summary information about the requestor’s qualification submissions, FDA’s formal written determinations and a listing of qualified biomarkers will be publicly posted.
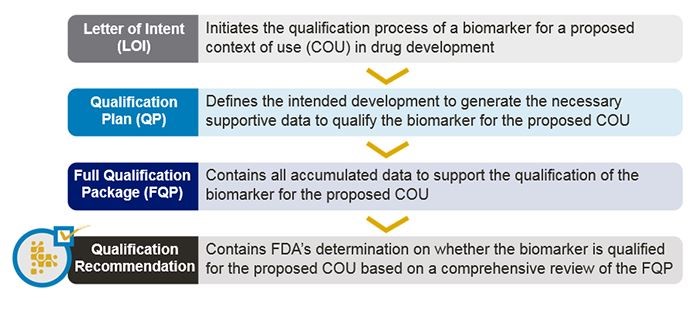
Stage 1: Letter of Intent (LOI)
A requestor submits the LOI in the recommended format. The LOI provides initial information about the biomarker proposal including:
- Drug development need the biomarker is intended to address
- Biomarker information
- Context of Use (COU)
- Information on how the biomarker will be measured
FDA will review the LOI submission to assess the biomarker’s potential value to address an unmet drug development need, as well as the proposal’s overall feasibility based upon current scientific understanding. If FDA accepts the LOI, the requestor may submit a Qualification Plan.
Stage 2: Qualification Plan (QP)
The QP is a detailed proposal describing the proposed biomarker development plan to provide the necessary information that will qualify the biomarker for the proposed COU in drug development. It summarizes existing information that supports the COU, identifies knowledge gaps, and proposes opportunities to address these gaps. The QP should include detailed information about the analytical method and performance characteristics.
If FDA accepts the QP, the agency will provide the requestor with instructions for the Full Qualification Package.
Stage 3: Full Qualification Package (FQP)
The FQP is a comprehensive compilation of supporting evidence that will inform the FDA’s qualification decision for the biomarker and COU. It contains all accumulated information, organized by topic area. FDA will make a final decision about whether the biomarker is qualified based on the FQP.
Upon qualification, the biomarker may be used under the COU for which it is qualified in any CDER drug development program to support the regulatory approval of a new drug.
- Additional Ways to Engage with CDER About Biomarker DevelopmentCritical Path Innovation Meeting (CPIM): a non-regulatory meeting where the requestor discusses and receives non-binding advice from CDER on topics related to how their proposed biomarker and context of use may enhance drug development.
- Letter of Support (LOS): a letter issued to a requestor that briefly describes CDER’s thoughts on the potential value of a biomarker and encourages further evaluation
Important Information for Requestors
- CDER & CBER’s DDT Qualification Project Search database
- Resources for Biomarker Requestors
- More About Biomarkers & Qualification
- General Biomarker Information
- 21st Century Cures Act
- Context of Use (COU)
- Biomarker FAQs
- BEST—a biomarker glossary
- Status of Biomarker Qualification Submissions
- Letter of Support
Contact us at: CDER-BiomarkerQualificationProgram@fda.hhs.gov