Context of Use
What is a context of use (COU) for a biomarker?
The COU is a concise description of the biomarker’s specified use in in drug development. The COU includes two components: (1) the BEST biomarker category and (2) the biomarker’s intended use in drug development. Each biomarker qualification effort should identify a single COU.
A COU is generally written to be consistent with the following structure:
[BEST biomarker category] to [drug development use].
Examples of Biomarker Intended Use in Drug Development
- Defining inclusion/exclusion criteria
- Defining treatment allocation arms
- Cessation of a patient’s participation in a clinical trial
- Establishing a drug’s proof of concept in a patient population
- Supporting clinical dose selection
- Serving to enrich clinical trial for an event or population of interest
- Evaluating treatment response
Note that a drug development use may also include descriptive information such as the patient population, disease or disease stage or model system; stage of drug development; and/or mechanism of action of the therapeutic intervention.
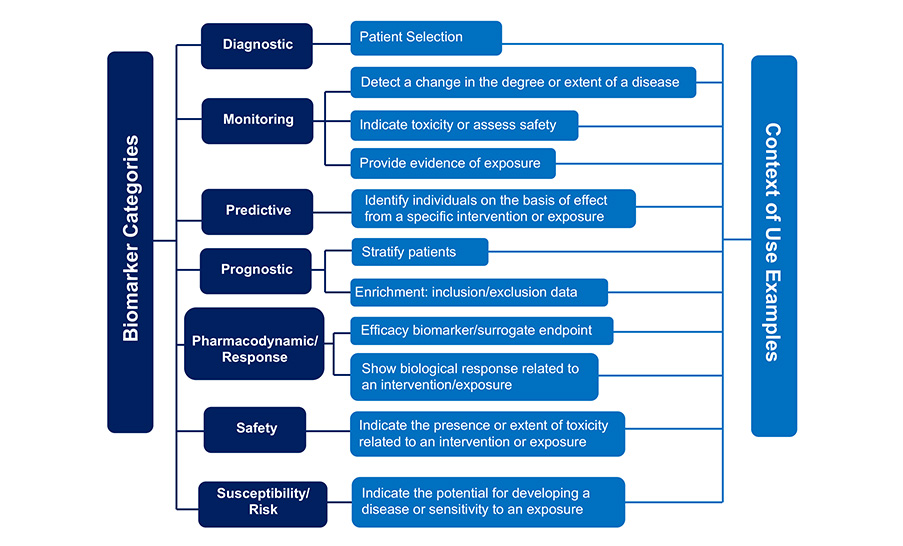
See the graphic below for examples of how a biomarker category and drug development use are related.
BEST Biomarker Category and Examples of Corresponding Drug Development Uses
Examples of COUs
- Predictive biomarker to enrich for enrollment of a sub group of asthma patients who are more likely to respond to a novel therapeutic in Phase 2/3 clinical trials.
- Prognostic biomarker to enrich the likelihood of hospitalizations during the timeframe of a clinical trial in phase 3 asthma clinical trials.
- Safety biomarker for the detection of acute drug-induced renal tubule alterations in male rats
Common COU Deficiencies to Avoid
To help requesters prepare more robust biomarker qualification submissions, the FDA has identified several recurring deficiencies in COUs that frequently delay regulatory review. Detailed information regarding these deficiencies and recommended approaches to avoid them is provided in the document linked below.
- Common COU Deficiencies to Avoid (PDF - 113 KB)
Important Information for Requestors
- CDER & CBER’s DDT Qualification Project Search database
- Resources for Biomarker Requestors
- About Biomarkers and Qualification
- More About Biomarkers & Qualification
- General Biomarker Information
- 21st Century Cures Act
- Context of Use (COU)
- Biomarker FAQs
- BEST—a biomarker glossary
- Status of Biomarker Qualification Submissions
- Letter of Support
Contact us at: CDER-BiomarkerQualificationProgram@fda.hhs.gov