Professional Education and Resources

In 2021, Project Socrates launched its second year, continuing many of its internal and external educational programs. Most notably, the FDA-AACR Oncology Educational Fellowship welcomed 29 new fellows to its 2021-2022 class in October 2021. Similar to its inaugural year, the Fellowship will span eight sessions over the course of a year and revisit Project ODAC Odyssey and many case discussions of approved oncology products.
OCE’s Project Livin’ Label now has five episodes available, each discussing the backstory of the development and FDA approval of an oncology product. In 2022, Project Livin’ Label welcomes the Oncology Nursing Society (ONS) and Hematology/Oncology Pharmacy Association (HOPA) as it looks to expand its scope by hearing the perspectives of oncology nurses and pharmacists.
Regarding outreach efforts, the FDA-ASCO Hematology/Oncology Fellows Workshop continued in its seventh year, with two half-day virtual sessions and almost 100 attendees at each session.
The OCE Icons in Oncology Distinguished Lecture Series welcomed Drs. James Allison, Martine Piccart, Judith Karp, and Lawrence Einhorn, who provided historical perspectives of how research, treatment paradigms, and clinical trials have evolved over time.
The Office of Oncologic Diseases welcomed 15 new clinical reviewers and analysts to the FDA oncology team, and Project Socrates held approximately 20 classes as part of the OCE Curriculum to educate new reviewers on oncology regulatory policy and science.
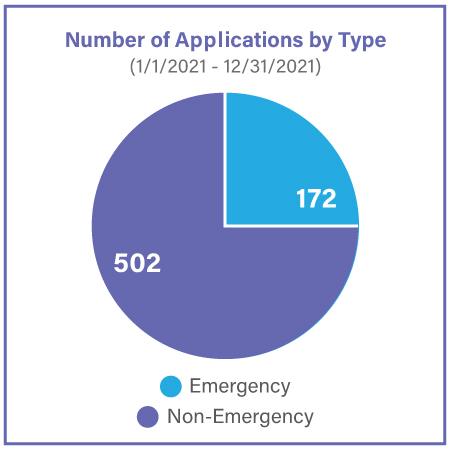
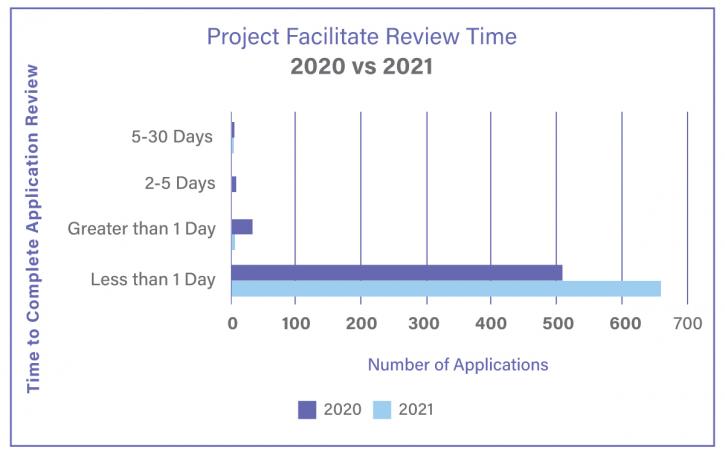
Project Facilitate is a program designed to assist oncology healthcare professionals in submitting Expanded Access requests to the FDA. In addition to providing support via phone or email, Project Facilitate staff also review all CDER oncology single-patient expanded access applications. In 2021, Project Facilitate handled 429 phone calls and 674 Expanded Access applications. Since Project Facilitate was launched, review and processing times have been reduced to less than one day, compared to two to three days on average prior to the program’s implementation. This is consistent with the Project Facilitate mission to improve efficiency of review of oncology Expanded Access requests. Project Facilitate staff also increased the number of educational outreach presentations on Expanded Access to clinics and hospital systems.
OCE’s Project Renewal is a public health initiative aimed at updating the labeling of long-standing, off-patent oncology drugs. Since its inception in 2018, Project Renewal has continued to refine its process of engaging a multi-disciplinary team of oncologists, clinical fellows in training, and other scientific experts to review older oncology drug labeling. This engagement culminates in an independent FDA review of clinical findings from published studies to ensure revised labeling provides adequate directions for use. In 2021, Project Renewal evaluated over 25 existing indications and potential off-label uses for three oncology drugs and fostered educational experiences for 12 hematology and 7 oncology fellows.
To date, Project Renewal has evaluated products which have a reference listed drug (RLD) New Drug Application (NDA) holder. In December 2020, the Making Objective Drug Evidence Revisions for New (MODERN) Labeling Act was passed, providing a regulatory framework for the FDA to update labeling of generic products approved under the abbreviated new drug applications pathway. Project Renewal, in collaboration with the FDA Office of Generic Drugs, looks forward to using the MODERN authority to revise important generic. oncology product labeling for cancer therapies that do not have an RLD NDA holder.
The OCE’s strong support for publications by FDA oncology/hematology staff continued in 2021, resulting in 86 articles in scientific journals. From 2010 through 2021, FDA oncology staff have published a total of 612 articles in scientific journals. OCE’s support for publications starts at the level of disease-specific team leaders who encourage and assist oncology reviewers in writing FDA approval summaries on most drug approvals. Staff are also encouraged to develop research articles on pooled analyses of clinical trial data. OCE and divisional leadership in the Office of Oncologic Diseases often write articles providing perspectives on topics in cancer drug development. The OCE Communications Team supports authors with editing and administrative services related to publication.