Risks and Complications of Breast Implants
Update: December 14, 2023
The FDA has updated the Risks and Complications of Breast Implants, clarifying information about Implant Removal and adding ultrasound as an acceptable alternative imaging for screening for rupture for asymptomatic patients to Silicone Gel-Filled Implant Rupture. The FDA also added a new subsection entitled, Reports of Squamous Cell Carcinoma (SCC), Various Lymphomas other than BIA-ALCL, and Mesenchymal Tumors, including Sarcoma.
FDA Strengthens Breast Implant Safety Requirements
On October 27, 2021, the FDA took several actions to strengthen breast implant risk communication and help those who are considering breast implants make informed decisions. These actions include orders restricting the sale and distribution of breast implants to help ensure that patients considering breast implants are provided with adequate risk information so that they can make fully informed decisions. These FDA requirements are described in detail at FDA Strengthens Safety Requirements and Updates Study Results for Breast Implants. In addition, the FDA regularly updates information on the status of breast implant manufacturers’ post-approval studies, at Post-Approval Studies (PAS) Database.
Risks of Breast Implants
Some of the complications and adverse outcomes of breast implants include:
- Implant complications, such as breast pain and changes in nipple and breast sensation
- Additional surgeries, with or without removal of the device (also see Implant Removal)
- Capsular contracture, scar tissue (capsule) that forms around the implant and squeezes the implant
- Rupture and deflation
- Breast implant associated-anaplastic large cell lymphoma (BIA-ALCL), a type of non-Hodgkin's lymphoma (cancer of the immune system)
- Reports of Squamous Cell Carcinoma (SCC), various lymphomas other than BIA-ALCL, and mesenchymal tumors, including sarcoma
- Connective tissue disease, breast cancer, and reproductive problems
- Systemic symptoms
- Impact on breastfeeding
- Effects on children
Implant Complications
The following is a list of local complications and adverse outcomes that occur in at least 1 percent of breast implant patients at any time. You may need non-surgical treatments or additional surgeries to treat any of these, and you should discuss any complication and necessary treatment with your doctor. These complications are listed alphabetically, not in order of how often they occur.
A complete list of complications, as well as information on rates for those complications can be found in the patient labeling for the approved breast implants, Labeling for Approved Breast Implants.
Additional Surgeries
Breast implants are not considered lifetime devices. The longer people have them, the greater the chances are that they will develop complications. Some complications will require more surgery. There is no guarantee that you will have a satisfactory cosmetic outcome from any reoperation.
The type of surgical procedure performed during a reoperation depends on the complication involved. You may need to have one or more reoperations over the course of your life due to one complication or a combination of local complications. More than one procedure may be performed in a single reoperation. Types of surgical procedures that may be performed in a reoperation include:
- Implant removal, with or without replacement
- Capsule removal (Capsulectomy) or surgical release of the scar tissue around the breast implant
- Scar or wound revision, such as surgical removal of excess scar tissue
- Drainage of a hematoma by inserting a needle or tube or the creation of a surgical skin incision to drain the collection of blood
- Repositioning of the implant by surgically opening the incision and moving the implant
- Biopsy/cyst removal by inserting a needle through the skin or cutting through the skin to remove a lump
Implant Removal
If you elect to have your breast implants removed, or if removal is medically indicated, there are two primary methods for implant removal. Your plastic surgeon may choose to remove your implant alone and leave the scar tissue that surrounds your implant in your body, also called the scar capsule. This option requires less surgical dissection and may pose less risk of local complications such as bleeding. Alternatively, your surgeon may also surgically remove the scar capsule when your breast implant is removed. This is called "en-bloc resection". You should discuss with your surgeon which method is best for your situation.
If you experience any symptoms of BIA-ALCL, such as persistent swelling or pain, or other changes in the area around your breast implant, talk to your surgeon or health care provider about the need for further evaluation. Evaluation for BIA-ALCL typically involves a physical exam, imaging, and/or assessment of the fluid or tissue around the breast implant. It is important to have an evaluation to diagnose BIA-ALCL because a confirmed BIA-ALCL diagnosis may change the type of operation that should be performed.
In the first method, your plastic surgeon may choose to remove your implant alone and leave the scar tissue that surrounds your implant in your body, -- also called the scar capsule – in your body. This option requires less surgical dissection and may pose less risk of local complications such as bleeding or damage to surrounding tissues.
In the second method, your surgeon may also surgically remove a part or all of the scar capsule when your breast implant is removed. This kind of surgery, which may be more extensive, is sometimes called "en-bloc resection," breast implant removal with “total capsulectomy” or “partial capsulectomy.” You should discuss with your surgeon which method is best for your situation or medical need.
Note that a confirmed diagnosis of breast implant associated-anaplastic large cell lymphoma (BIA-ALCL) may change the type of operation that should be performed. If you experience any symptoms of BIA-ALCL, such as persistent swelling or pain, or other changes in the area around your breast implant, talk to your surgeon or health care provider about the need for further evaluation. Evaluation for BIA-ALCL typically involves a physical exam, imaging, and/or assessment of the fluid or tissue around the breast implant.
Generally, patients with confirmed BIA-ALCL should undergo implant removal and removal of the surrounding scar capsule, which is a more extensive operation than implant removal alone. Talk to your surgeon about the method of removal most appropriate for you.
The life of breast implants varies by person and cannot be predicted. You may need to have your implant removed at some time over the course of your life because of one or more complications. With any surgery, there may be risks associated with the surgery itself or anesthesia. Talk to your surgeon about the benefits and risks of surgical options before making an informed decision.
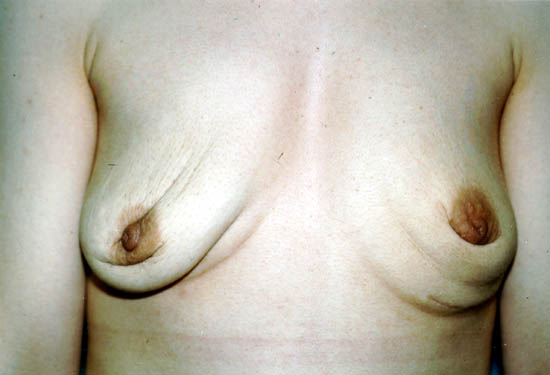
After removal, some women do not choose to replace their implants. These women may have cosmetically undesirable dimpling, chest wall concavity, puckering, or sagging of their natural breasts.
The photograph below shows a 29-year-old woman 1 year after having her silicone gel-filled breast implants removed, but not replaced. Women with large breast implants, especially those inserted on top of the chest muscles (subglandularly), may have major cosmetic deformity if they choose not to replace them or to undergo additional reconstructive surgery.
Photo courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C., University of Toronto.
Some insurance companies do not cover implant removal or implant replacement, even if there are complications and even if the first implant surgery was covered.
Capsular Contracture
Capsular contracture is the hardening of the breast around the implant. It can occur in the tissue surrounding one or both implants. This hardening causes the tissue to tighten, which can be painful.
Capsular contracture may be more common following infection, hematoma and seroma. However, the cause of capsular contracture is not known.
There are four grades of capsular contracture, known as Baker grades.
Baker Grading Scale
- Grade I: Breast is normally soft and looks natural
- Grade II: Breast is a little firm but looks normal
- Grade III: Breast is firm and looks abnormal
- Grade IV: Breast is hard, painful, and looks abnormal
Grades III and IV capsular contracture are considered severe and may require reoperation. The surgical procedure usually involves removal of the implant with or without replacement of the implant. Capsular contracture could occur again after surgery to correct it.
The FDA has not cleared or approved any devices to treat or reduce the incidence of capsular contracture.
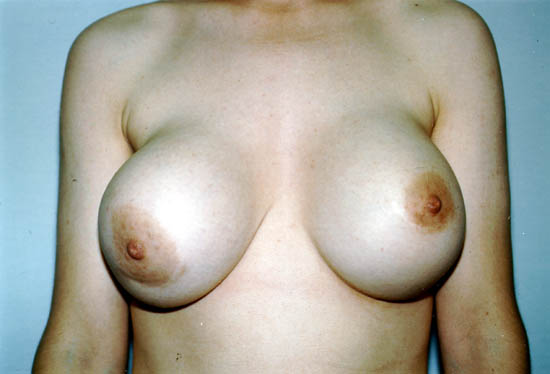
The picture below shows a Grade IV capsular contracture in the right breast of a 29-year-old woman 7 years after placement of silicone gel-filled breast implants.
Photo courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C., University of Toronto.
Rupture and Deflation
Rupture is a tear or hole in the outer shell of the breast implant.
Some possible causes of rupture of breast implants include:
- Capsular contracture
- Compression during a mammogram
- Damage by surgical instruments
- Damage during procedures to the breast, such as biopsies and fluid drainage
- Normal aging of the implant
- Overfilling or underfilling of saline-filled breast implants
- Physical stresses such as trauma or intense physical pressure
- Placement through a non-FDA approved incision site, for example the belly button
- Too much handling during surgery
Saline-Filled Breast Implant Rupture and Deflation
The term "rupture" is used for all types of breast implants, but the term deflation is only used for saline-filled implants. You and/or your doctor will be able to tell if your saline-filled implant ruptures because the saline solution leaks into your body immediately or over several days and deflates the implant. You will notice that your implant loses its original size or shape.
The following surgical procedures are not recommended for FDA-approved saline-filled breast implants because they are known to cause rupture and deflation:
- Closed capsulotomy - a technique used to relieve capsular contracture involving manually squeezing the breast to break the hard capsule
- Placement of drugs or substances other than sterile saline inside the implant Any contact of the implant with Betadine, a povidone-iodine topical antiseptic made by Purdue Frederick Company
- Any contact of the implant with Betadine, a povidone-iodine topical antiseptic made by Purdue Frederick Company
- Injection through the implant shell
- Alteration of the implant
- Stacking of the implants (more than one implant per breast pocket)
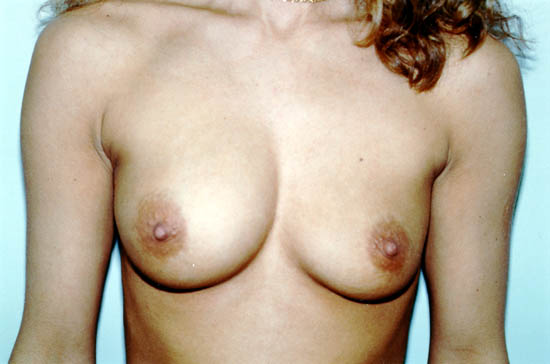
The photograph below shows a 30-year-old woman whose left saline-filled breast implant deflated. The implant is thought to have deflated due to a particular design, which is no longer used by the manufacturer.
Photo courtesy of Walter Peters, Ph.D., M.D., F.R.C.S.C., University of Toronto.
Silicone Gel-Filled Implant Rupture
Silicone breast implants can rupture at any time after your implant surgery, but the longer an implant is in place, the greater the possibility an implant may rupture.
If a silicone gel-filled breast implant ruptures, it is not likely that you or your doctor will immediately notice because most silicone implant ruptures are without symptoms, known as "silent ruptures." A silent rupture usually does not change the way an implant looks or feels, and your surgeon or health care provider may not be able to detect a silent rupture by a physical examination alone. Magnetic resonance imaging (MRI) is the most effective method for detecting silent rupture of silicone gel-filled breast implants. An ultrasound is an acceptable alternative imaging for screening for rupture for asymptomatic patients.
Occasionally when a silicone gel-filled implant ruptures, you may notice a decrease in breast size, change in breast implant shape, hard lumps over the implant or chest area, an uneven appearance of the breasts, pain or tenderness, tingling, swelling, numbness, burning, or changes in sensation.
Generally, when silicone gel-filled implants rupture, the silicone gel escapes through a tear or hole in the implant shell but remains confined within the scar tissue around the implant, called an intra-capsular rupture. If the gel migrates beyond the scar tissue around the breast implant, it is called an extracapsular rupture. Sometimes, after a rupture, the gel may move to other distant areas around the body. This is called extracapsular rupture with gel migration. It may be difficult to remove silicone gel after a rupture.
Connective Tissue Disease, Breast Cancer, and Reproductive Problems
The FDA has not detected any association between silicone gel-filled breast implants and connective tissue disease, breast cancer or reproductive problems. However, the FDA has received reports of systemic symptoms (see below) by some patients with both saline and silicone gel-filled breast implants. In order to fully understand these complications, studies would need to be larger and longer than those conducted so far.
Systemic Symptoms [Breast Implant Illness (BII)]
Symptoms such as fatigue, memory loss, rash, "brain fog," and joint pain may be associated with breast implants. Some patients may use the term "breast implant illness" (BII) to describe these symptoms. Researchers are investigating these symptoms to better understand their origins. These symptoms and what causes them are poorly understood. In some cases, removal of the breast implants without replacement is reported to reverse symptoms of breast implant illness.
We encourage patients to report any injury, adverse event, or symptom related to a medical device, including the symptoms listed above, to the FDA by phone at 1-800-FDA-1088 or online at MedWatch, the FDA Safety Information and Adverse Event Reporting program. Please include the following information:
- Device Name (Brand Name)
- Manufacturer's Name
- Details of Adverse Event and Medical and/or Surgical Interventions (if applicable)
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and Reports of Squamous Cell Carcinoma (SCC), Various Lymphomas other than BIA-ALCL, and Mesenchymal Tumors including Sarcoma
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a T-cell lymphoma that can develop following breast implants. For additional information, see: Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL).
Reports of Squamous Cell Carcinoma (SCC), Various Lymphomas other than BIA-ALCL, and Mesenchymal Tumors, including Sarcoma
Scientific literature and Medical Device Reports include reports of Squamous Cell Carcinoma (SCC) and various lymphomas other than BIA-ALCL in the scar tissue around breast implants. For additional information, see: UPDATE: Reports of Squamous Cell Carcinoma (SCC) in the Capsule Around Breast Implants - FDA Safety Communication.
The FDA has also become aware of reports of various mesenchymal tumors, including sarcomas of the breast, in patients with breast implants. The FDA will continue to gather and review all available data to evaluate the occurrence of various malignancies of the breast in patients with breast implants.
Understanding Patients’ Preferences
Patients’ voices, preferences and perspectives are critical to understanding the impact medical devices have on their conditions and their quality of life.
The FDA’s Center for Devices and Radiological Health (CDRH) is studying how patients weigh the benefits and risks associated with smooth and textured breast implants including the risk of Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL), which appears more often in patients with textured breast implants. CDRH contracted with Research Triangle Institute (RTI) International to conduct this study. To ensure the study prioritized the needs and experiences of patients, CDRH and RTI designed the survey tool based on input from focus groups made up of patients considering, or having had, breast reconstruction or augmentation using breast implants, as well as input from health care providers.
CDRH expects the survey results will help assess whether patients’ perception of risks of BIA-ALCL associated with textured breast implants are influenced by information about potential benefits of textured breast implants.
The insights gained through this patient-centered approach will help incorporate patient preferences in CDRH’s regulatory decision-making. CDRH is working to publish the results of this study in a peer-reviewed publication. We will continue to update the public when more information is available.
Impact on Breastfeeding
Some women who undergo breast augmentation can successfully breastfeed and some cannot. Women who undergo mastectomies and then have breast implant reconstruction surgeries may not be able to breastfeed on the affected side due to loss of breast tissue and the glands that produce milk.
Effects on Children
At this time, it is not known if a small amount of silicone may pass through from the breast implant silicone shell into breast milk during breastfeeding. Although there are currently no established methods for accurately detecting silicone levels in breast milk, a study measuring silicon (one component in silicone) levels did not indicate higher levels in breast milk from women with silicone gel-filled implants when compared to women without implants.
In addition, concerns have been raised regarding potential damaging effects on children born to mothers with implants. Two studies in humans have found no increased risk of birth defects in children born to mothers who have had breast implant surgery. Although low birth weight was reported in a third study, other factors (for example, lower pre-pregnancy weight) may explain this finding.