Multimedia Education Materials | Biosimilars
FDA offers a variety of materials in multiple formats and languages to help promote understanding of biosimilars and interchangeable products. Explore the available resources FDA has for a variety of audiences, including patients, caregivers, and health care providers. Patient and health care provider materials are available in Arabic (عربي), French (Français), Haitian Creole (Kreyòl ayisyen), Korean (한국인), Simplified Chinese (简体中文), Spanish (Español), Tagalog, Traditional Chinese (繁體中文), and Vietnamese (Tiếng Việt).
Multimedia Education Materials for Patients
Fact Sheets
Infographics
عربي (Arabic)
- المُتشابهات الحيويّة: ما يحتاج المرضى إلى معرفته
(Biosimilars: What Patients Need to Know, Fact Sheet) - لمُتشابهات الحيويّة: ما يحتاج مرضى السُكري إلى معرفته
(Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet) - معلومات أساسيّة عن المُتشابهات الحيويّة
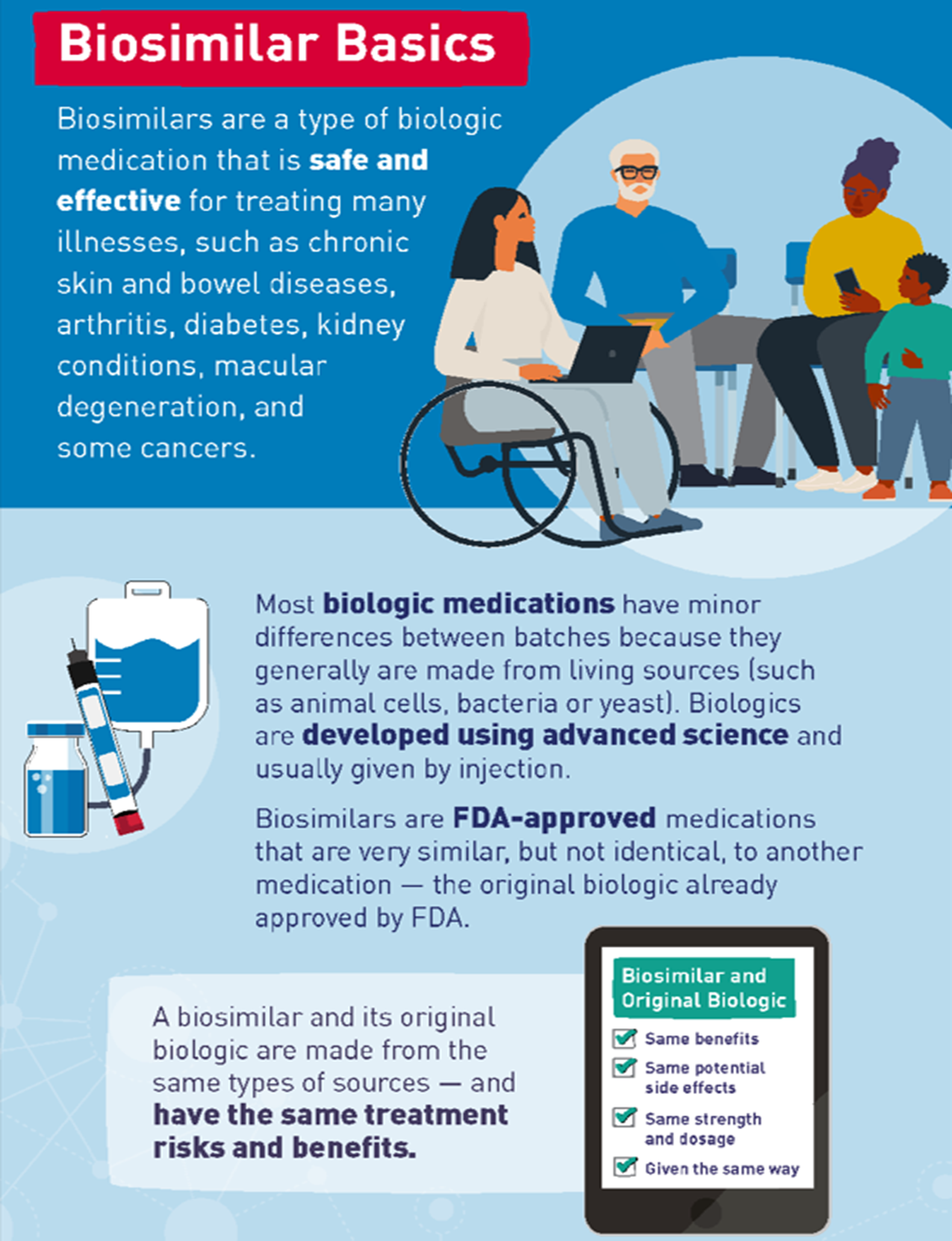
(Biosimilars Basics, Infographic)
Français (French)
- Les médicaments biosimilaires: Ce que les patients doivent savoir (Biosimilars: What Patients Need to Know, Fact Sheet)
- Les médicaments biosimilaires: Ce que les patients atteints du diabète doivent savoir (Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet)
- Les principes de base des médicaments biosimilaires (Biosimilar Basics, Infographic)
Kreyòl ayisyen (Haitian Creole)
- Byosimilè : sou ki sa pasyan yo dwe konnen (Biosimilars: What Patients Need to Know, Fact Sheet)
- Byosimilè : Ki sa Pasyan ki fè Dyabèt yo Dwe Konnen (Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet)
- Prensip Fondamantal yon Byosimilè (Biosimilar Basics, Infographic)
한국인 (Korean)
- 바이오시밀러: 환자들이 알아야 할 것 (Biosimilars: What Patients Need to Know, Fact Sheet)
- 바이오시밀러: 당뇨병 환자들이 알아야 할 것 (Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet)
- 바이오시밀러 기초 (Biosimilar Basics, Infographic)
简体中文 (Simplified Chinese)
- 生物类似药:患者需知 (Biosimilars: What Patients Need to Know, Fact Sheet)
- 生物类似药:糖尿病患者须知 (Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet)
- 生物类似药基础知识 (Biosimilar Basics, Infographic)
- 您是否在使用生物药品? (Are you on a biologic medication?, Fact Sheet)
Español (Spanish)
- Biosimilares: Lo que los pacientes deben saber (Biosimilars: What Patients Need to Know, Fact Sheet)
- Biosimilares: Lo que los pacientes con diabetes deben saber (Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet)
- Conceptos básicos sobre los biosimilares (Biosimilar Basics, Infographic)
- ¿Está usted tomando un medicamento biológico? (Are you on a biologic medication?, Fact Sheet)
Tagalog
- Mga Biosimilar: Mga Kailangang Malaman ng mga Pasyente (Biosimilars: What Patients Need to Know, Fact Sheet)
- Mga Biosimilar: Mga Kailangang Malaman ng mga Pasyenteng May Diabetes (Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet)
- Mga Basic sa Biosimilar (Biosimilar Basics, Infographic)
繁體中文 (Traditional Chinese)
- 生物相似性藥品:病人需知 (Biosimilars: What Patients Need to Know, Fact Sheet)
- 生物相似性藥品:糖尿病病人須知 (Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet)
- 生物相似性藥品基礎知識 (Biosimilar Basics, Infographic)
- 您是否在使用生物藥品? (Are you on a biologic medication?, Fact Sheet)
Tiếng Việt (Vietnamese)
- Thuốc tương tự sinh học: Điều Bệnh nhân Cần Biết (Biosimilars: What Patients Need to Know, Fact Sheet)
- Thuốc tương tự sinh học: Điều Bệnh nhân Mắc bệnh Tiểu đường Cần Biết (Biosimilars: What Patients with Diabetes Need to Know, Fact Sheet)
- Thông tin cơ bản về Thuốc tương tự sinh học (Biosimilar Basics, Infographic)
We hope that you find these translations useful. While the agency has attempted to obtain translations that are as faithful as possible to the English version, we recognize that the translated versions may not be as precise, clear, or complete as the English version. The official version of these translations is the English version.
Videos for Patients
Consumer Update
Read the FDA’s Consumer Update to learn more about biosimilars
Multimedia Education Materials for Health Care Providers
Fact Sheets
عربي (Arabic)
- نظرة عامة على المُتشابهات الحيويّة
(Overview of Biosimilar Products) - المُراجعة التنظيميّة للمُتشابهات الحيويّة واعتمادها
(Biological Regulatory Review and Approval) - المُنتجات الحيويّة المقبولة الاستبدال
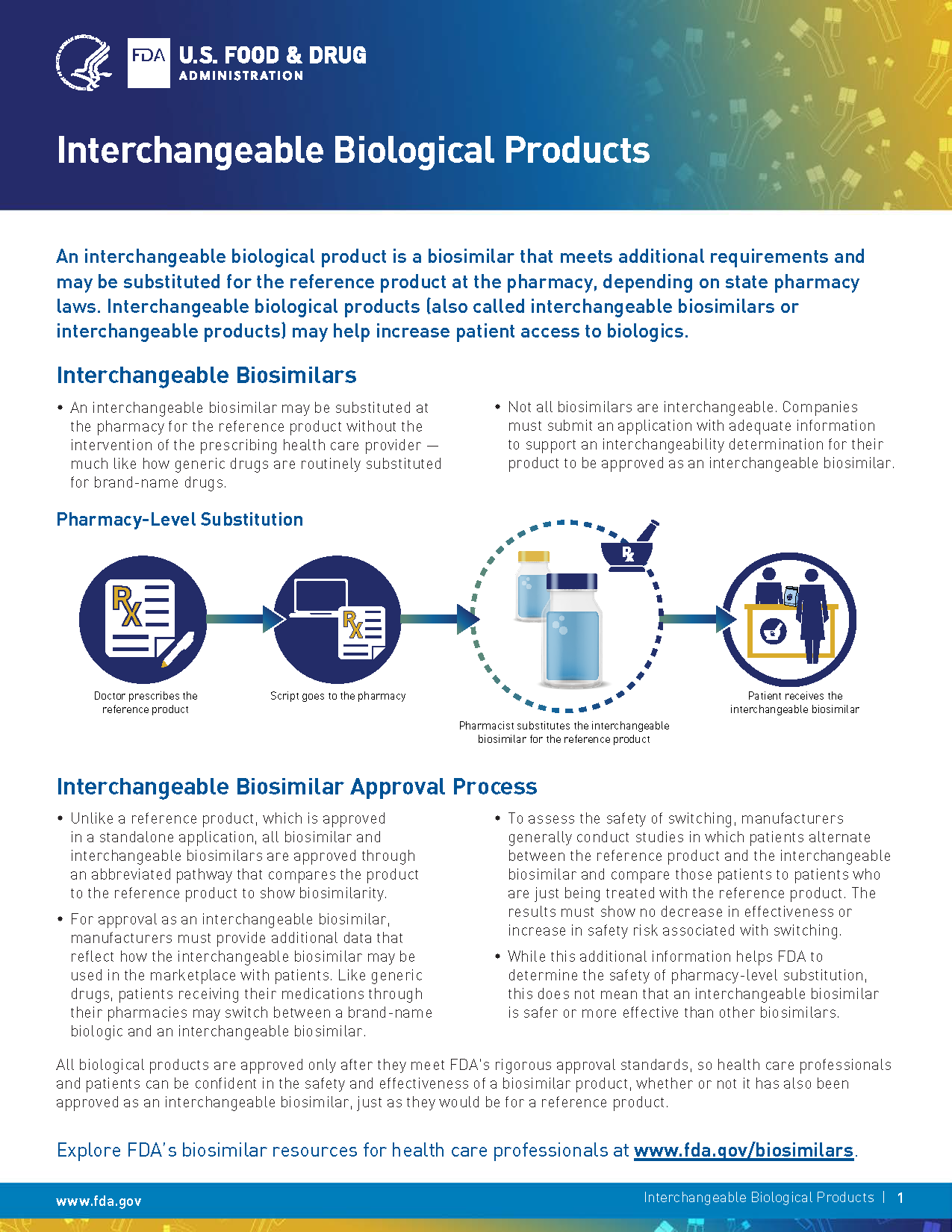
(Interchangeable Biological Products)
Français (French)
- Aperçu des Médicaments Biosimilaires (Overview of Biosimilar Products)
- Examen et autorisation biologiques réglementaires (Biological Regulatory Review and Approval)
- Médicaments biologiques interchangeables (Interchangeable Biological Products)
Kreyòl ayisyen (Haitian Creole)
- Yon Rale sou Pwodui Byosimilè yo (Overview of Biosimilar Products)
- Revizyon ak Apwobasyon Reglemantè Pwodui Byolojik (Biological Regulatory Review and Approval)
- Pwodui Byolojik Entèchanjab (Interchangeable Biological Products)
한국인 (Korean)
- 바이오시밀러 제품의 개요 (Overview of Biosimilar Products)
- 생물학적 규제 심사 및 승인 (Biological Regulatory Review and Approval)
- 대체 가능한 생물학적 제품들 (Interchangeable Biological Products)
简体中文 (Simplified Chinese)
- 生物类似药产品概述 (Overview of Biosimilar Products)
- 生物监管审批 (Biological Regulatory Review and Approval)
- 可互换生物制品 (Interchangeable Biological Products)
Español (Spanish)
- Visión general de los productos biosimilares (Overview of Biosimilar Products)
- El proceso de aprobación y revisión reglamentaria de biosimilares (Biological Regulatory Review and Approval)
- Productos biológicos intercambiables (Interchangeable Biological Products)
Tagalog
- Pangkalahatang-ideya sa mga Biosimilar na Produkto (Overview of Biosimilar Products)
- Pagsusuri at Pag-apruba ng mga Biologic para sa Regulasyon (Biological Regulatory Review and Approval)
- Mga Interchangeable na Biological na Produkto (Interchangeable Biological Products)
繁體中文 (Traditional Chinese)
- 生物相似性藥品概述 (Overview of Biosimilar Products)
- 生物監管審查和批准 (Biological Regulatory Review and Approval)
- 可互換的生物製劑 (Interchangeable Biological Products)
Tiếng Việt (Vietnamese)
- Tổng quan về các Chế phẩm Tương tự sinh học (Overview of Biosimilar Products)
- Hoạt động Xem xét và Phê duyệt Sinh học của Cơ quan quản lý (Biological Regulatory Review and Approval)
- Các Chế phẩm Sinh học Có thể hoán đổi (Interchangeable Biological Products)
We hope that you find these translations useful. While the agency has attempted to obtain translations that are as faithful as possible to the English version, we recognize that the translated versions may not be as precise, clear, or complete as the English version. The official version of these translations is the English version.
Infographics
Videos for Health Care Providers
- Interchangeable Biosimilars (Duration: 1m 35s | YouTube)
Continuing Education Activities
These continuing education, FDA-developed activities provides healthcare providers the knowledge and understanding they need to speak to their patients about biosimilars. Visit the Medscape Biosimilar Homepage to access the available courses, or click the individual course links to go directly to that activity
Please note: a Medscape login is required to access these activities.
- The Road to Biosimilars in Rare Hematologic Conditions (medscape.org)
- Biosimilars in Rheumatology: Building Certainty and Reducing Reluctance (medscape.org)
- Biosimilars in Multiple Sclerosis: Are We Ready? (medscape.org)
- Overcoming Reluctance and Enhancing Biosimilar Adoption (medscape.org)
- Biosimilars for the Management of Eye Conditions (medscape.org)
- Developing a Plan of Care That Includes Biosimilars When Uncertainty Is Present (medscape.org)
- Biosimilars 102: Interchangeability, Extrapolation, and Immunogenicity -- A Regulatory Process Primer (medscape.org)
- Biosimilars in the Real World: Perspectives for Staying Within the Scope of Care
- Biosimilars 101: A Primer for Your Practice
- Test Your Skill: Incorporating Biosimilars Into the Management of Patients With Immunological Conditions
- Putting the Patient Into Perspective: Strategies for Educating Patients About Biosimilars