Review and Approval
All FDA-approved biological products (biologics), including biosimilars and interchangeable biosimilars, undergo a rigorous evaluation so that health care providers and patients can be confident of the safety, effectiveness, and quality of these products.
Learn more about the review and approval processes for biosimilars and interchangeable biosimilars:
- What is the approval process for biosimilar products?
- Why do we need an abbreviated approval pathway for biological products?
- What data are required for approval of a biosimilar product?
- Can a biosimilar be approved for an indication that is approved for the reference product even if the biosimilar is not directly studied in that indication?
- Are there additional data requirements for interchangeable biosimilar products?
What is the approval process for biosimilar products?
A biosimilar is a biologic that is highly similar to and has no clinically meaningful differences in terms of safety, purity, and potency (safety and effectiveness) from an existing FDA-approved biologic, called a reference product. A reference product is a biological product that has been approved in a stand-alone application that contains all data to demonstrate the product’s safety and effectiveness for each of the indications being sought by the manufacturer and is the product against which a proposed biosimilar is evaluated. Biosimilars are evaluated for approval based on all the evidence presented by the manufacturer.
FDA approves biosimilars through an abbreviated pathway. The goal of a biosimilar development program is to demonstrate biosimilarity between the proposed biosimilar and its reference product, not to independently establish the safety and effectiveness of the proposed biosimilar. This generally means that biosimilar manufacturers do not need to conduct as many expensive and lengthy clinical trials.
Learn more about variation in manufacturing biological products in relation to the biosimilar approval process.
In this webinar, FDA provides an intermediate overview of the scientific concepts about biological products and the scientific and regulatory basis for the biosimilar pathway.
Why do we need an abbreviated approval pathway for biological products?
- Biologics are the fastest-growing class of medications in the United States and account for a substantial and growing portion of health care costs.
- The Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway to help provide patients with greater access to safe and effective biological products. This pathway helps reduce the time and cost of development without compromising safety and effectiveness.
- Learn more about how biosimilars can facilitate price competition and patient access.
What data are required for approval of a biosimilar product?
FDA evaluates each proposed biosimilar individually and advises manufacturers on the scope and extent of testing needed to show biosimilarity.
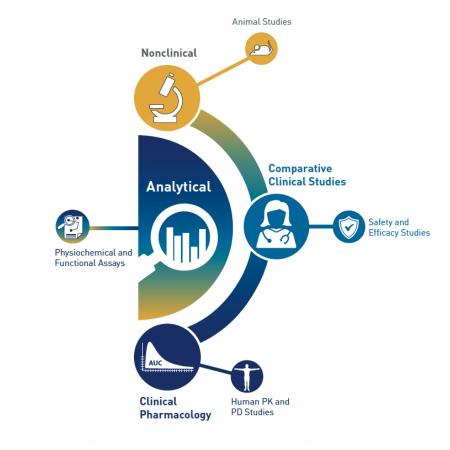
An application for a proposed biosimilar generally must include information showing that the proposed product is biosimilar to a reference product. Below is some information on the data typically included in an application. More information can be found here.
- Analytical studies. Comparative analytical data provide the foundation of biosimilar development. These studies provide data to support the structural and functional similarity of the proposed product to the reference product and to evaluate the impact of any differences identified.
- Animal studies. These may provide toxicology or pharmacology information.
- A clinical study or studies. Pharmacology studies may demonstrate that the proposed biosimilar moves through the body in the same way and provides the same effects as the reference product. An immunogenicity assessment evaluates a patient’s immune response to the proposed biosimilar. Other comparative clinical studies are sometimes conducted after the completion of other studies to address any remaining uncertainty about whether the proposed biosimilar has any clinically meaningful differences from the reference product. Learn more about comparative clinical studies.
FDA has discretion to determine that an element is unnecessary in a proposed biosimilar application.
FDA evaluates all the evidence, based on comparisons between the biosimilar and the reference product, in the context of the Agency’s previous finding that the reference product is safe and effective.
Learn about FDA’s industry information and guidance on biologics and biosimilars.
Regulatory and Scientific Concepts
Level 2 in the curriculum for health care degree programs provides a more in-depth look at scientific and regulatory topics related to biosimilars and their practical applications.
Can a biosimilar be approved for an indication that is approved for the reference product even if the biosimilar is not directly studied in that indication?
A biosimilar can meet the requirements for approval, in part, based on data from a clinical study or studies that demonstrate safety and effectiveness in an appropriate condition of use. Therefore, FDA may approve a biosimilar for indications or populations without direct clinical studies in those indications or populations if the manufacturer provides adequate scientific justification, based on factors such as:
- All available information in the biosimilar application
- FDA’s determination of the safety and efficacy of the reference product for the approved indications
- Knowledge and consideration of various scientific factors for each indication
Are there additional data requirements for interchangeable biosimilar products?
An interchangeable biosimilar is a biosimilar that may be substituted for the reference product without the intervention of the prescribing health care provider, depending on state pharmacy laws. Not all biosimilars are interchangeable biosimilars. A manufacturer must specifically seek FDA approval for an interchangeable product.
The approval process for interchangeable biosimilars has additional requirements related to the potential for substitution. Patients receiving their medications through their pharmacies may switch between a brand-name biological product and an interchangeable biosimilar. This is similar to how generic drugs can be substituted for brand-name drugs at the pharmacy.
In addition to establishing biosimilarity, a manufacturer of an interchangeable biosimilar needs to submit information to show that the proposed product can be expected to produce the same clinical result as the reference product in any given patient.
Also, for certain interchangeable biosimilars, FDA evaluates information on the effects of switching between the reference product and the interchangeable biosimilar. Therefore, manufacturers typically conduct a switching study, in which patients alternate between the reference product and the interchangeable biosimilar, and then compare those patients to patients who are treated with only the reference product.
While this additional information helps FDA to determine if a biosimilar is an interchangeable biosimilar, this does not mean that an interchangeable biosimilar is safer or more effective than other biosimilars. Both biosimilar and interchangeable biosimilars meet the same high standards for quality and similarity to the reference product. All biological products are approved only after they meet FDA’s rigorous approval standards.
- Download these fact sheets for health care professionals: Biosimilar Regulatory Review and Approval and Interchangeable Biological Products