COMPANY ANNOUNCEMENT
Zydus Pharmaceuticals (USA) Inc. Issues Voluntary Nationwide Recall of Acyclovir Sodium Injection, 50 mg/mL Due to Crystallization
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to Crystalization
- Company Name:
- Zydus Pharmaceuticals Inc.
- Brand Name:
-
Brand Name(s)Zydus Pharmaceuticals
- Product Description:
-
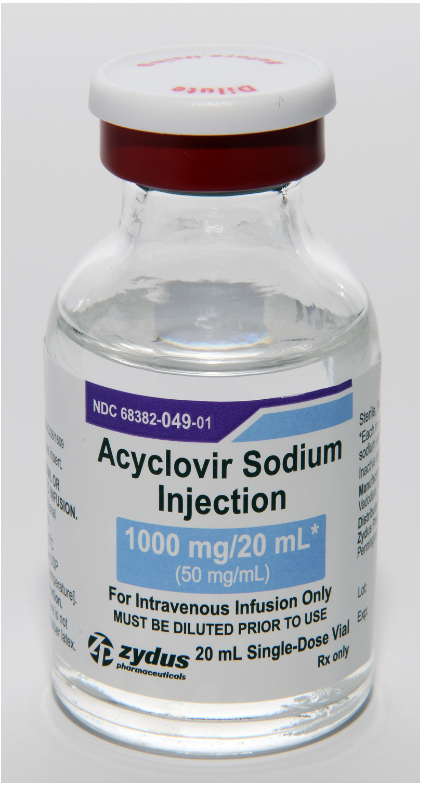
Product DescriptionAcyclovir Sodium Injection, 50 mg/mL, 10 mL and 20 mL vials
Company Announcement
Pennington, NJ, Zydus Pharmaceuticals (USA) Inc. is voluntarily recalling four lots of Acyclovir Sodium Injection, 50 mg/mL, 10 mL and 20 mL vials, to the Hospital/User level after receiving several complaints of crystallization in vials.
Administration of crystalized Acyclovir Sodium Injection, 50 mg/mL has a potential of life-threatening adverse consequences including injection site inflammation of a vein and local reactions, damage and/or obstruction of blood vessels, which could induce clots, particularly in the lungs, the passage of the particulate matter into the bloodstream may lead to clots resulting in stroke, heart attack, decreased liver or kidney function or death of tissues or cells. To date, Zydus Pharmaceuticals (USA) Inc. has not received any reports of adverse events related to this product recall.
Acyclovir Sodium Injection, 50 mg/mL is indicated for the treatment of herpes simplex infections in immunocompromised patients, severe initial clinical episodes of herpes genitalis in immuno-competent patients, herpes simplex encephalitis, neonatal herpes simplex virus infection and varicella-zoster (shingles) infections in immunocompromised patients. The product is packaged in single-dose glass vials and was distributed nationwide in the USA to Cardinal Health, Amerisourcebergen Drug Corporation and Morris & Dickson Company LLC. The affected Acyclovir Sodium Injection, 50 mg/mL lots include the following lot numbers and expiration dates:
| Product | Carton NDC Number | Vial NDC Number | Lot Number | Expiry Date | Pack Size |
|---|---|---|---|---|---|
| Acyclovir Sodium Injection, 50 mg/mL, 20 mL |
68382-049-10 | 68382-049-01 | L000155 | Dec 2021 | 10x20 mL, Single-Dose Vial pack |
| Acyclovir Sodium Injection, 50 mg/mL, 20 mL |
68382-049-10 | 68382-049-01 | L000156 | Jan 2022 | 10x20 mL, Single-Dose Vial pack |
| Acyclovir Sodium Injection 50 mg/mL, 10 mL |
68382-048-10 | 68382-048-01 | L000126 | Dec 2021 | 10x10 mL, Single-Dose Vial pack |
| Acyclovir Sodium Injection 50 mg/mL, 10 mL |
68382-048-10 | 68382-048-01 | L000127 | Dec 2021 | 10x10 mL, Single-Dose Vial pack |
Zydus Pharmaceuticals (USA) Inc. has notified its distributors and customers by email and FedEx overnight courier service and is arranging for the return of all recalled Acyclovir Sodium Injection, 50 mg/mL lots. Hospitals that have the product which is being recalled should stop using it immediately and call our recall coordinating center at 1-855-671-5023 Monday – Friday (excluding holidays), 9:00 am to 5:00 pm EST.
Consumers with questions regarding this recall can contact Zydus Pharmaceuticals (USA) Inc. by phone: 1-877-993-8779 or by email: medicalaffairs@zydususa.com Monday – Friday (excluding holidays), 9:00 am to 5:00 pm EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Zydus Pharmaceuticals (USA) Inc.
- 1-877-993-8779
- medicalaffairs@zydususa.com