COMPANY ANNOUNCEMENT
Viona Pharmaceuticals Inc., Issues Voluntary Nationwide Recall of Metformin HCl Extended- Release Tablets, USP 750 mg, Due to the Detection of N-Nitrosodimethylamine (NDMA) Impurity
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionN-Nitrosodimethylamine (NDMA) Impurity
- Company Name:
- Viona Pharmaceuticals, Inc.
- Brand Name:
-
Brand Name(s)Viona
- Product Description:
-
Product DescriptionMetformin Hydrochloride Extended-Release Tablets
Company Announcement
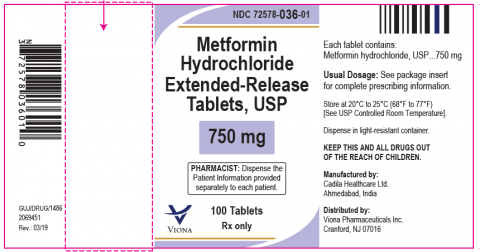
FOR IMMEDIATE RELEASE – 01/07/2022 – Cranford, New Jersey, Viona Pharmaceuticals Inc., is voluntarily recalling twenty-three (23) lots of Metformin Hydrochloride Extended-Release Tablets, USP 750 mg at the consumer level. The reason for the recall is an Out of Specification (OOS) result observed for one lot of the product (M008132) “N-nitrosodimethylamine (NDMA) (By GC- MS/MS)” test at 17 Month(s), 25°C/60%RH Long-term stability samples. In an abundance of caution, the firm has decided to voluntarily recall 23 batches which we have determined having a valid shelf life within the US market. This product was manufactured by Cadila Healthcare Limited, Ahmedabad, India for U.S. distribution by Viona Pharmaceuticals Inc.
Risk Statement: NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables. Patients who have received impacted lots of Metformin Hydrochloride Extended-Release Tablets, USP 750 mg are advised to continue taking their medication and contact their physician for advice regarding an alternative treatment. According to the FDA, it could be dangerous for patients with this serious condition to stop taking their Metformin without first talking to their healthcare professionals. Please visit the agency’s website for more information at https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press- announcements-ndma-metformin. To date, neither Viona Pharmaceuticals Inc., nor Cadila Healthcare Limited have received any reports of adverse events related to this recall.
The product is used as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus and is packaged in HDPE bottles of 100 tablets, under NDC 72578-036-01. The recalled lots of Metformin Hydrochloride Extended-Release Tablets, USP 750 mg are listed in the below table. The product can be identified as white to off-white, capsule shaped, uncoated tablets, debossed with "Z", "C" on one side and "20" on the other side. Metformin Hydrochloride Extended-Release Tablets, USP 750 mg was distributed Nationwide to Distributors.
| Product Name: Metformin Hydrochloride Extended‐Release Tablets, USP 750 mg NDC: 72578‐036‐01 |
||
| Sr. No. | Batch No. | Exp. Date |
|---|---|---|
| 1. | M008130 | 06/2022 |
| 2. | M008131 | 06/2022 |
| 3. | M008132 | 06/2022 |
| 4. | M008133 | 06/2022 |
| 5. | M010080 | 07/2022 |
| 6. | M010081 | 07/2022 |
| 7. | M011029 | 08/2022 |
| 8. | M011030 | 08/2022 |
| 9. | M011031 | 08/2022 |
| 10. | M011032 | 08/2022 |
| 11. | M011304 | 08/2022 |
| 12. | M013394 | 09/2022 |
| 13. | M013395 | 09/2022 |
| 14. | M013396 | 09/2022 |
| 15. | M013966 | 09/2022 |
| 16. | M013967 | 09/2022 |
| 17. | M100831 | 12/2022 |
| 18. | M100832 | 12/2022 |
| 19. | M100833 | 01/2023 |
| 20. | M100834 | 01/2023 |
| 21. | M101267 | 01/2023 |
| 22. | M102718 | 01/2023 |
| 23. | M102719 | 01/2023 |
Viona Pharmaceuticals Inc., is notifying its customers by email and mail (FedEx Overnight) and is arranging for the return of all recalled products to our recall processor at the following address
Inmar Pharmaceuticals Services-Recalls
3845 Grand Lakes Way,
Grand Prairie, Texas 75050.
Consumers with questions regarding this recall can contact our recall processor Inmar Pharmaceutical Services by phone at 1-855-249-3303, option 1; Monday – Friday (excluding holidays), 9:00 am – 5:00 pm, EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Customers with medical-related questions, who wish to report an adverse event, or quality issues about the products being recalled should contact Viona Pharmaceuticals Inc., by phone at: 888-304-5011, Monday - Friday, 8:30 am – 5:30 pm, EST.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Inmar Pharmaceutical Services
- 1-855-249-3303