COMPANY ANNOUNCEMENT
Urgent Medical Device Recall: Sam XT Extremity Tourniquet
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
General Hospital & Personal Use - Reason for Announcement:
-
Recall Reason Descriptionpossible failure of the stitches securing the buckle to the nylon belt
- Company Name:
- SAM Medical
- Brand Name:
-
Brand Name(s)SAM
- Product Description:
-
Product DescriptionExtremity Tourniquets
Company Announcement
SAM Medical today announced it is conducting a voluntary international recall of all unused SAM XT Extremity Tourniquets (SAM XT). The company initiated the recall after internal testing indicated a possible failure of the stitches securing the buckle to the nylon belt could occur, posing a potential risk when used on a human patient to stop arterial blood flow. To date, there have been no reports of adverse health consequences received. This recall is being made with the knowledge of the Food and Drug Administration and other relevant Competent Authorities.
Product Identification
The recall involves all unused SAM XTs manufactured with the multi-pass straight lockstitch (Fig A.2), distributed from March 2017 through April 2018, with the following lot numbers. The lot number is located on the face of the buckle.
| PART NUMBER | MODEL | LOT NUMBERS w/ multi-pass straight lockstitch (see Fig A.2) |
|---|---|---|
| SAM XT-M | Tactical Black or Military | XT1711 thru XT1811 |
| SAM XT-C | Hi-Viz Orange or Civilian | XT1711 thru XT1811 |
| SAM XT-B | Hi-Viz Blue | XT1808 thru XT1811 |
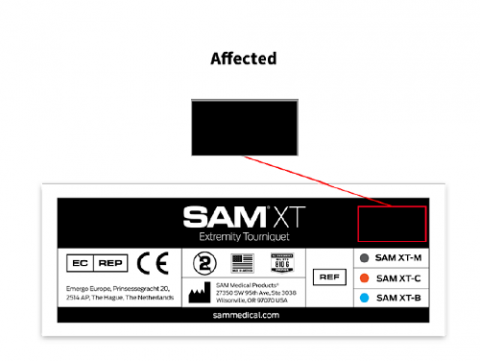
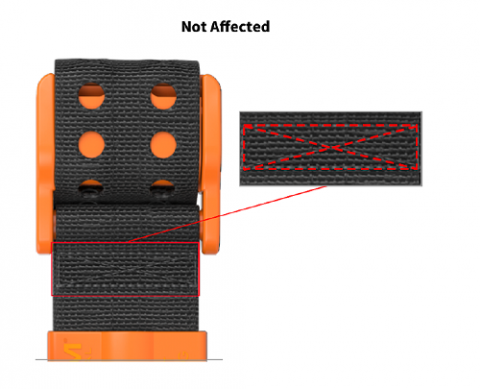
To help identify whether you have a tourniquet that is subject to the recall, in addition to the lot numbers in the prior table, please read Figures A.1 and Figure A.2 below.
Figure A.1
An affected tourniquet will not have the “Box X Stitch” icon on the upper right of the folded Instructions For Use (IFU) insert. Tourniquets not affected will display the “Box X Stitch” icon on the upper right of the folded IFU.
Figure A.2
An affected tourniquet will have a multi-pass straight lockstitch. Tourniquets not affected will have a “Box X” stitch (stitching is highlighted in red for display purposes only).
Remedy
All SAM XTs are now being manufactured with a “Box X” stitch which produces an inherently stronger stitch pattern. In addition, the company initiated more extensive simulated-use testing to ensure the revised stitching process is consistently reliable. Production and replacement of all recalled SAM XTs with the improved stitching is currently underway.
Notifications Made
Concurrent with this press release, SAM Medical is notifying all SAM XT distributors and direct sales customers by email and signature-required postage. Each customer will receive instructions on how to arrange for a return of all recalled product. Customers and distributors must return all unused affected product through their distribution channel.
If you purchased product directly from SAM Medical:
- Immediately examine your inventory and quarantine product subject to recall pursuant to the identification instructions above.
- Immediately discontinue use and/or distribution of any affected products.
- If you received a recall information packet from SAM Medical, follow the instructions in that packet for return of the recalled product.
- If you have not received an information packet from SAM Medical by May 16, 2018, please contact the company at xtrecall@sammedical.com.
If you purchased product from a party other than SAM Medical:
- Immediately examine your inventory and quarantine product subject to recall pursuant to the identification instructions above.
- Immediately discontinue use and/or distribution of any affected products.
- Contact the seller of the product and ask for instructions for return of all unused SAM Medical XTs through that distributor.
- If you do not have information on where you purchased the SAM XT, please contact the company at xtrecall@sammedical.com
Customers with questions may contact the company at +1 800- 580-3519 between the hours of 8:00 a.m. and 5:00 p.m. PT. Customers may also contact the company by email at xtrecall@sammedical.com or through the website at www.sammedical.com/xtrecall.
About SAM Medical
For over 30 years, SAM Medical has developed and manufactured innovative medical products used for military, law enforcement, emergency, wilderness and sports medicine, and pre-hospital care around the world. A resounding favorite of medical professionals, SAM Medical's lineup of products is engineered to preserve life. Innovations include SAM XT Extremity Tourniquet, SAM Splint, SAM Chest Seal, SAM Junctional Tourniquet, SAM Pelvic Sling, ChitoSAM, and SAM Soft Shell Splint. For more information, visit sammedical.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.