COMPANY ANNOUNCEMENT
Universal Meditech Inc. Issues Nationwide Recall of Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test Kit
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason DescriptionProducts were distributed without appropriate premarket clearance or approval which potentially could result in inaccurate test results due to lack of performance evaluation by the FDA.
- Company Name:
- Universal Meditech Inc.
- Brand Name:
-
Brand Name(s)Skippack Medical Lab, DiagnosUs
- Product Description:
-
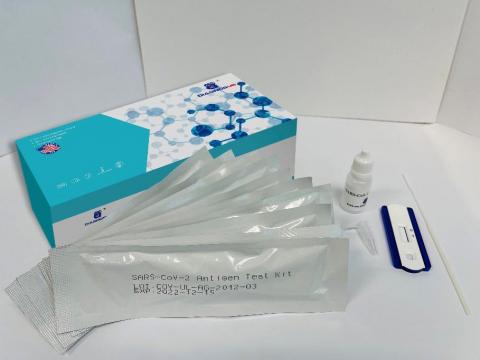
Product DescriptionSARS-CoV-2 Antigen Rapid Test Kits
Company Announcement
On Dec 29, 2022, Universal Meditech Inc. initiated a nationwide recall of 56,300 Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test Kits. The product(s) have been found to have been distributed without appropriate premarket clearance or approval which potentially could result in inaccurate test results due to lack of performance evaluation by the FDA.
A recall of the same device has been previously conducted by SML Distribution LLC, for which the details can be found at the link below:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=193010
Consumers who have Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test Kits should stop using the device immediately and contact the distributor for product return.
Recalled products were manufactured from October 2021 to December 2021 and distributed in January 2022.
The following styles/models/UDI have been recalled:
- Name of Product: Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test Kit
- UDI: None
- Model: Cassette
- Quantity: 56,300
Products were distributed with “Skippack Medical Lab” branded Instructions for Use leaflet in three different packaging boxes identified below:
- Purple and white box under “Skippack Medical Lab” brand: (see image below)
- Green and white box under “DiagnosUS” brand: (see image below)
- White box without brand name: (see image below)
Universal Meditech Inc. voluntarily recalled the product after becoming aware of the violative distribution notified by the FDA.
To date, there has not been any reported injury.
Universal Meditech Inc. is notifying its distributors and customers by phone and email and is arranging for return of all recalled products.
Universal Meditech Inc. has distributed the products to distributors in California and Texas.
Consumers with questions may contact the legal attorney of the company via telephone at +1(702)871-9888 between the hours of 9AM and 5PM, P.S.T. Consumer may also contact the legal attorney of the company via e-mail at ml@linlawgroup.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Company Contact Information
- Consumers:
- legal attorney of the company
- +1-702-871-9888
- ml@linlawgroup.com