COMPANY ANNOUNCEMENT
Teva Issues Voluntary Nationwide Recall of One Lot of IDArubicin Hydrochloride Injection USP 5 mg/5 mL Due to the Presence of Particulate Matter
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionPotential Particulate Matter (silica and iron oxide)
- Company Name:
- Teva Pharmaceuticals
- Brand Name:
-
Brand Name(s)Teva Pharmaceuticals
- Product Description:
-
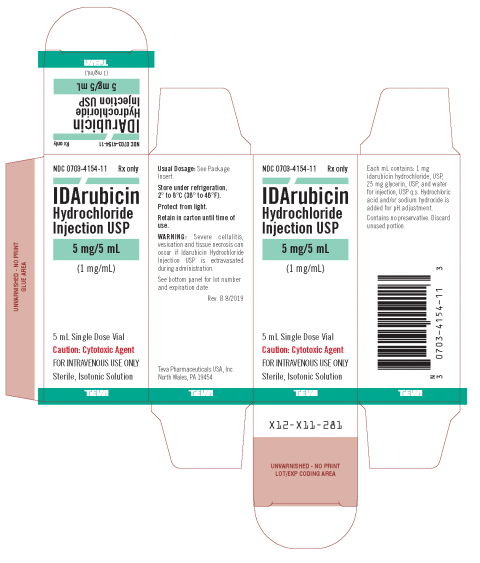
Product DescriptionIDArubicin Hydrochloride Injection USP
Company Announcement
Teva Pharmaceuticals has initiated a voluntary nationwide recall of lot 31329657B of IDArubicin Hydrochloride Injection USP 5 mg/5 mL vial, to the user level in the United States. This voluntary recall is initiated based on an internal inspection that found particulate matter in one vial of the product identified as silica and iron oxide. No other vials have been observed to contain this defect. To date, Teva has received no product quality complaints or adverse event reports of this nature for the subject recall lot.
The administration of an injectable product that contains particulate matter may result in local irritation or swelling in response to the foreign material. If the particulate matter reaches the blood vessels it can travel to various organs and block blood vessels in the heart, lungs or brain which can cause stroke and even lead to death. While the health hazard risk could be severe if particulate matter is infused, Teva’s internal health assessment determined that the likelihood of patient harm is remote or unlikely
IDArubicin Hydrochloride Injection USP in combination with other approved anti-leukemic drugs is indicated for the treatment of acute myeloid leukemia (AML) in adults. Information about the affected product is listed in the table below. It is packed in 5 mL Single Dose Vials. Teva distributed 1,565 vials Nationwide from 12-04-2020 through 08-18-2021 to 4 of its Wholesale customers under the label for Teva Pharmaceuticals USA, Inc.
| Vial/Carton NDC | Lot # | Exp. Date |
|---|---|---|
| 0703-4154-11 | 31329657B | 08/2023 |
Teva notified its customers on March 28, 2022 and asked that the lot be recalled and to make arrangements for impacted product to be returned. Instructions for returning recalled product and crediting are given in the recall letter released by Teva.
Any consumer who has questions or concerns should first consult with their health care provider(s). To report an Adverse Event or Quality Complaint, or have Medical Related Questions, please use the following contact information:
Medical-related Questions or to report an Adverse Event:
Contact Medical Information at: 888-838-2872 , option 3, then, option 4
Live calls received: M - F, 9:00 AM - 5:00 PM Eastern Time; Voicemail: 24 hrs./day, 7 days/week
24 hrs. /day, 7 days/week or by email at druginfo@tevapharm.com.
Product Quality Complaint-related Questions:
Contact Quality Assurance Services: 888-838-2872, option 4
Live calls received: M - F, 9:00 AM - 5:00 PM Eastern Time; Voicemail: 24 hrs./day, 7 days/week
Adverse reactions or other problems experienced with the use of these products may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall was made with the knowledge of the Food and Drug Administration. Teva will continue to partner with, and regularly update, all relevant stakeholders, including regulatory authorities, to resolve this situation.
Company Contact Information
- Consumers:
- Contact Medical Information
- 888-838-2872
- druginfo@tevapharm.com