COMPANY ANNOUNCEMENT
Sleepnet Corporation Issues Worldwide Recall of CPAP and BIPAP Masks with Magnets Due to Potential Interference with Certain Medical Implants
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason DescriptionPotential interference with certain medical implants
- Company Name:
- Sleepnet Corporation
- Brand Name:
-
Brand Name(s)Mojo, Mojo 2, iQ 2, Phantom 2
- Product Description:
-
Product DescriptionCPAP and BIPAP Masks with Magnets
Company Announcement
Hampton, NH – On March 1, 2024, Sleepnet Corporation initiated a worldwide recall for all CPAP and BIPAP masks with magnets due to potential interference with certain medical devices. When a magnet comes into close proximity to certain medical implants or metallic implants, it could interfere with the performance or the position of the implant, potentially resulting in serious injury or death.
Sleepnet has been distributing masks with magnets worldwide since 2006 and to date, there have been no Medical Device Reports associated with the Sleepnet masks with magnets. This is a voluntary action based on information obtained from post market surveillance.
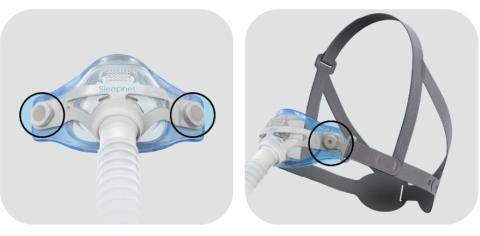
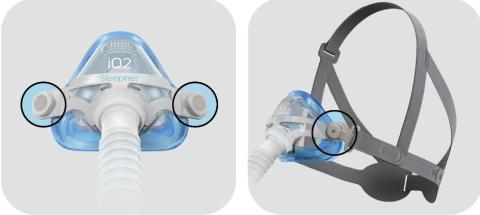
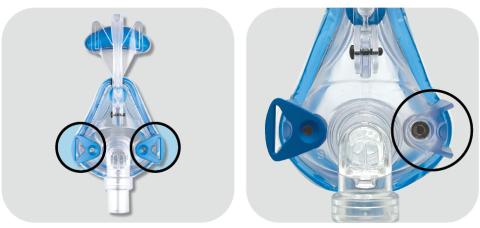
Affected products are Mojo Full Face Vented Mask, Mojo Full Face Non‐Vented Mask, Mojo 2 Full Face Vented Mask, Mojo 2 Full Face Non‐ Vented Mask, Mojo 2 Full Face AAV Non‐Vented Mask, iQ 2 Nasal Mask, and Phantom 2 Nasal Mask. This is applicable to all lot/UDI numbers.
If a patient or anyone (example: household members, bed partners, caregivers, etc.) in close physical contact with the mask has an active medical implant or metallic implant that will interact with magnets, these masks with magnets should not be used. Implant examples include, but are not limited to, pacemakers, implantable cardioverter defibrillators (ICD), neurostimulators, aneurysm clips, metallic stents, ocular implants, insulin/infusion pumps, cerebral spinal fluid (CSF) shunts, embolic coils, metallic splinter, implants to restore hearing or balance with implanted magnets (such as cochlear implants), flow disruption devices, contact lenses with metal, dental implants, metallic cranial plates, screws, burr hole covers, bone substitute device, magnetic metallic implants/electrodes/valves placed in upper limbs , torso, or higher, etc.
Sleepnet masks with magnets are safe when used in accordance with the newly updated Instructions for Use. Continue using masks according to the updated instructions and labeling if patients or people in close proximity to them do not have implanted metallic medical devices or metallic objects in the body.
Sleepnet is adding a new contraindication and an updated warning to the Instructions for Use labeling of the affected products. This will be included in the labeling for all future production of these masks. The labeling will be updated to state the following:
New Contraindication
Do not use this mask if you or anyone (example: household members, bed partners, caregivers, etc.) in close physical contact with your mask has an active medical implant or metallic implant that will interact with magnets. Implant examples include, but are not limited to, pacemakers, implantable cardioverter defibrillators (ICD), neurostimulators, aneurysm clips, metallic stents, ocular implants, insulin/infusion pumps, cerebral spinal fluid (CSF) shunts, embolic coils, metallic splinter, implants to restore hearing or balance with implanted magnets (such as cochlear implants), flow disruption devices, contact lenses with metal, dental implants, metallic cranial plates, screws, burr hole covers, bone substitute device, magnetic metallic implants/electrodes/valves placed in upper limbs , torso, or higher, etc. If you have any questions regarding the implant, consult your physician or the manufacturer of your implant.
Updated Warning
Magnets are used in the mask and headgear clips with a field strength of 380mT. With the exception of the devices identified in the contraindication, ensure that the mask is kept at least 6 inches (approx. 16 cm) away from any other medical implants or medical devices that can be impacted by the magnetic fields to avoid possible effects from localized magnetic fields. This applies to you or anyone in close physical contact with your mask.
If a patient, or anyone in close physical contact, has an active medical implant or metallic implant, they should contact their mask supplier to find a replacement mask that does not include magnets. If the patient is unsure whether or not they should use the mask, they should consult their physician or the manufacturer of their implant.
Consumers with questions may contact Sleepnet at hkoppusetty@sleepnetcorp.com or call 1‐800‐742‐3646 between the hours of 8:00 AM – 4:00 PM EST.
Sleepnet has notified the FDA and other global regulators where required.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
About Sleepnet
We believe in building products that help people lead healthier lives. Our passion for innovation is at the core of everything we do, laying a foundation for creating new ideas and building better products. As a company with BAA‐ compliant products, we are experienced manufacturers focused on developing best‐in‐class products that improve the health and well‐being of our customers. For more than 25 years, Sleepnet has manufactured gel masks and respirators built to help customers breathe easier. Our products include Continuous Positive Airway Pressure masks, Noninvasive Ventilation masks, Pediatric masks, and Respirators. With comfort and safety top of mind, we proudly design and manufacture our products in the United States. To learn more, visit www.sleepnetmasks.com.